Guided Notes Section 7 - Morrison Community Unit District 6
advertisement
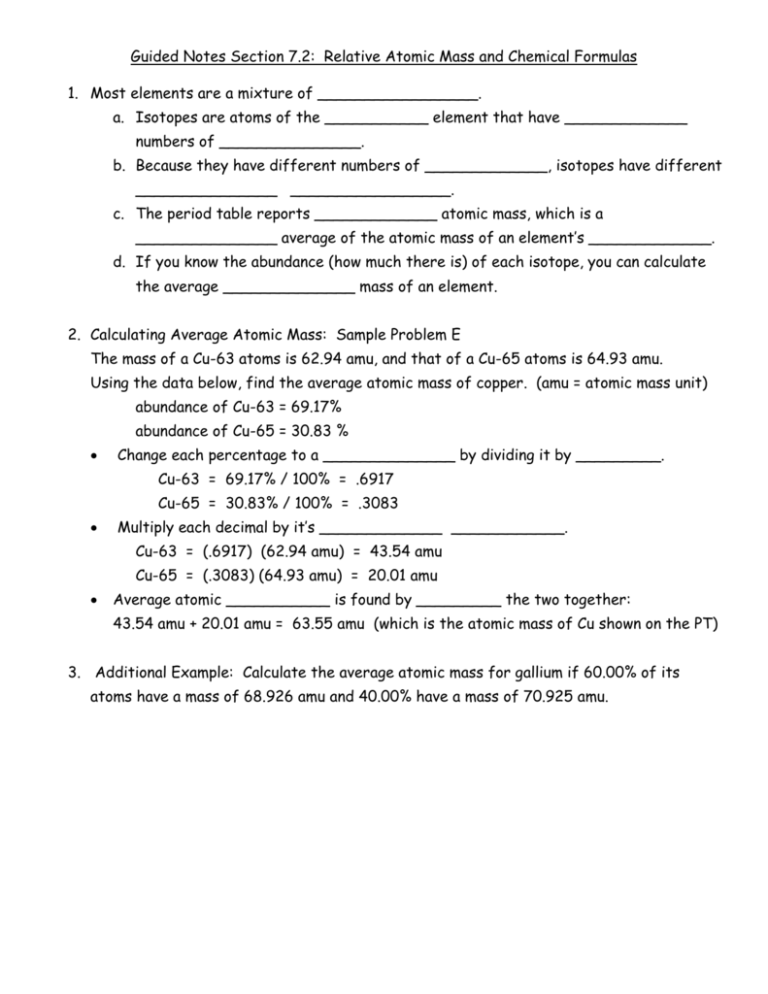
Guided Notes Section 7.2: Relative Atomic Mass and Chemical Formulas 1. Most elements are a mixture of _________________. a. Isotopes are atoms of the ___________ element that have _____________ numbers of _______________. b. Because they have different numbers of _____________, isotopes have different _______________ _________________. c. The period table reports _____________ atomic mass, which is a _______________ average of the atomic mass of an element’s _____________. d. If you know the abundance (how much there is) of each isotope, you can calculate the average ______________ mass of an element. 2. Calculating Average Atomic Mass: Sample Problem E The mass of a Cu-63 atoms is 62.94 amu, and that of a Cu-65 atoms is 64.93 amu. Using the data below, find the average atomic mass of copper. (amu = atomic mass unit) abundance of Cu-63 = 69.17% abundance of Cu-65 = 30.83 % Change each percentage to a ______________ by dividing it by _________. Cu-63 = 69.17% / 100% = .6917 Cu-65 = 30.83% / 100% = .3083 Multiply each decimal by it’s _____________ ____________. Cu-63 = (.6917) (62.94 amu) = 43.54 amu Cu-65 = (.3083) (64.93 amu) = 20.01 amu Average atomic ___________ is found by _________ the two together: 43.54 amu + 20.01 amu = 63.55 amu (which is the atomic mass of Cu shown on the PT) 3. Additional Example: Calculate the average atomic mass for gallium if 60.00% of its atoms have a mass of 68.926 amu and 40.00% have a mass of 70.925 amu. 4. A compound’s chemical formula tells you which ____________, as well as __________ much of each, are present in a compound. a. Formulas for ______________ compounds show the elements and the number of ___________ of each element in a molecule. b. Formulas for _____________ compounds show the ______________ ratio of ______________ and ____________ in any pure sample. 5. Formulas for _______________ ions show the _______________ ratio of cations and anions. a. They also show the ______________ and the number of ___________ of each element in each ion. b. For example, the formula KNO3 indicates a ratio of one ________ cation to one __________ anion. 6. Calculating Molar Mass of Compounds: Sample Problem F Find the molar mass of barium nitrate. Barium is a ________ cation and nitrate is a _________ anion. You need ________ nitrate ions to balance with the _________ barium cation. The simplest formula is: ___________________ To find the molar mass of Ba(NO3)2, ________ the atomic masses of each __________ together: Barium: 137.33 g/mol = 137.33 g/mol Nitrogen: 2 (14.01 g/mol) = 28.02 g/mol Oxygen: 6 (16.00 g/mol) = 96.00 g/mol Molar mass of Ba(NO3)2 = 261.35 g/mol 7. Additional Practice: Calculate the molar mass of (NH4)2SO3









