Atoms-Ions-and-Molecules
advertisement

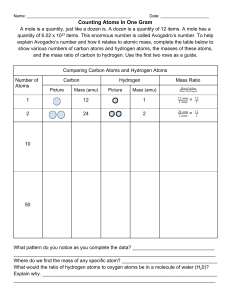
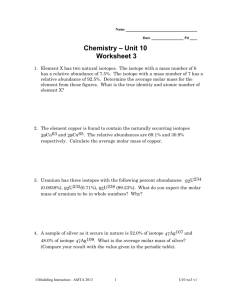
Atoms, Ions and Molecules – Calculations 1. How do you calculate the atomic mass of an element? a. What is the atomic mass of copper if 63Cu has a mass of 62.93 amu and percent abundance of 69.09% and isotope 65Cu has a mass of 64.93 amu and percent abundance of 30.91%. b. What are the percent abundances for 151Eu and 153Eu if the atomic mass of the element is 151.96 amu and the isotope masses are 150.9196 amu and 152.9209 amu respectively. 2. What is a mole? 3. How does the molecular formula relate to the number of moles? 4. What is the molar mass of a compound? a. Calculate the molar mass of C6H6. 5. In 3.35 x 1022 total atoms of CH3OH there are how many a. Molecules b. Moles c. Grams 6. What is % composition (by mass)? 7. Fungal laccase is 0.390% Cu by mass. If a fungal laccase molecule contains 4 copper atoms, what is the molar mass of fungal laccase? 8. _____________ is a technique frequently used to determine the formula of an unknown substance (typically a hydrocarbon). When using this experimental method the ____________ formula is obtained – this is the formula with the most __________ _______ between atoms). Using this and the actual molar mass of the compound a ___________ formula can be determined. 9. Combustion requires the presence of ___________. 10. HxCy + O2 _________ + __________ 11. Terephthalic acid contains C, H and O. Combustion of 19.81 mg terephthalic acid produces 41.98 mg CO2 and 6.45 mg H2O. If 0.250 mol of terephthalic acid has a mass of 41.5g, determine the molecular formula of terephthalic acid.