Aldol Condensation: Dibenzalacetone Synthesis Lab Manual
advertisement

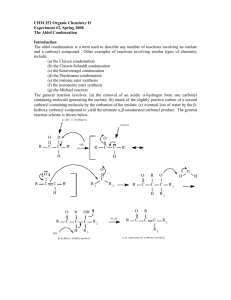
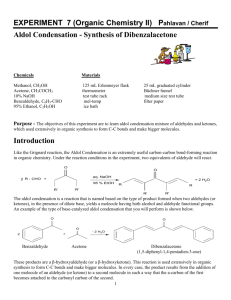
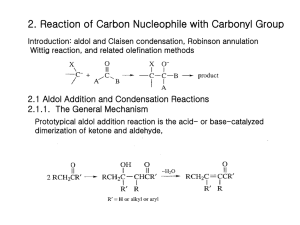
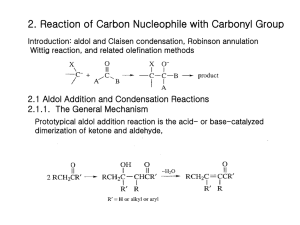
EXPERIMENT 7 (Organic Chemistry II) Pahlavan/Cherif Aldol Condensation - Synthesis of Dibenzalacetone Chemicals Materials Methanol, CH3OH Acetone, CH3COCH3 10% NaOH Benzaldehyde, C6H5-CHO 95% Ethanol, C2H5OH Hot water bath ice bath large tube 10 ml graduated cylinder Buchner funnel Büchner funnel thermometer mel-temp Purpose - The objectives of this experiment are to learn aldol condensation mixture of aldehydes and ketones, which used extensively in organic synthesis to form C-C bonds and make bigger molecules. Introduction T Like the Grignard reaction, the Aldol Condensation is an extremely useful carbon-carbon bond-forming reaction in organic chemistry. Under the reaction conditions in the experiment, two equivalents of aldehyde will react. The aldol condensation is a reaction that is named based on the type of product formed when two aldehydes (or ketones), in the presence of dilute base, yields a molecule having both alcohol and aldehyde functional groups. An example of the type of base-catalyzed aldol condensation that you will perform is shown below. Reaction Scheme O O 2 O + H Benzaldehyde H3C - 2 H2O CH3 Acetone Dibenzalacetone (1,5-diphenyl-1,4-pentadien-3-one) These products are a β-hydroxyaldehyde (or a β-hydroxyketone). This reaction is used extensively in organic synthesis to form C-C bonds and make bigger molecules. In every case, the product results from the addition of one molecule of an aldehyde (or ketone) to a second molecule in such a way that the a-carbon of the first becomes attached to the carbonyl carbon of the second. 1 MECHANISM OF THE ALDOL CONDENSATION The acidity of the alpha-carbon makes beta-dehydration of aldols an easy reaction. (This is of course quite different than the chemistry of normal alcohols.) This conjugated enone synthesis is catalyzed by both acids and bases. This shows the mechanism of the experiment performed. The reaction proceeds by an aldol condensation. Step 1: First, an acid-base reaction. Hydroxide functions as a base and removes the acidic -hydrogen giving the reactive enolate. Step 2: The nucleophilic enolate attacks the aldehyde at the electrophilic carbonyl C in a nucleophilic addition type process giving an intermediate alkoxide. Step 3: An acid-base reaction. The alkoxide deprotonates a water molecule creating hydroxide and the hydroxyaldehydes or aldol product. MECHANISM OF THE DEHYDRATION OF AN ALDOL PRODUCT Step 1: First, an acid-base reaction. Hydroxide functions as a base and removes an acidic -hydrogen giving the reactive enolate. Step 2: The electrons associated with the negative charge of the enolate are used to form the C=C and displace the leaving group, regenerating hydroxide giving the conjugated aldehyde. 2 Dehydration generally occurs under slightly more vigorous conditions, such as higher temperature, than the condensation reaction. Thus at higher temperature in base the aldol reaction will go directly to the conjugated enone without any isolation of the aldol intermediate. The aldehyde and ketone used for aldol reaction will be selected from the following group: Notice that none of the aldehydes shown above contains an enolizable -hydrogen, so they cannot act as the nucleophilic species in the aldol reaction. Ketones, in general, are less susceptible to nucleophilic attack than aldehydes, so in a reaction mixture containing both an aldehyde and a ketone, the aldehyde will react faster with nucleophiles. Thus, it is possible to perform a "crossed" aldol reaction in which the enolate formed by abstraction of the alpha-hydrogen on the ketone attacks the carbonyl of the aldehyde. Since we are working with conjugated aldehydes, the resulting beta-hydroxyketones readily eliminate water to form enones. Under the conditions used in this experiment (an excess of aldehyde), a "double condensation" occurs by reaction on both sides of the ketone to give the products shown below. Although a β-hydroxyaldehyde (or a β-hydroxyketone) is produced in an aldol condensation, the ultimate product of these reactions (as shown above) is often the α,β-unsaturated aldehyde and a separate molecule of water. Upon heating, the β-hydroxy aldehyde product of an aldol condensation easily undergoes dehydration to yield an α,β-unsaturated aldehyde (or ketone). Conjugation of the newly formed double bond with the carbonyl group (and of the benzene ring, as shown in the example below) stabilizes the product and provides the thermodynamic driving force for the dehydration process. In the present case, the reaction—a mixed, or crossed aldol condensation involving an aromatic aldehyde—is referred to as a Claisen-Schmidt condensation. The Claisen-Schmidt condensation always involves dehydration of the product of the mixed addition to yield a product in which the double bond (produced during dehydration) is conjugated to both the aromatic ring and the carbonyl group. 3 Because this aromatic aldehyde lacks a-hydrogens, only one product is formed, rather than a mixture of four different compounds, as long as the concentration of the second aldehyde is carefully controlled. In this experiment we will prepare the dibenzalacetone: 1,5-diphenyl-1,4-pentadien-3-one. The equilibrium is shifted toward the product because the compound precipitates from the reaction mixture as it is formed. 1,5-diphenyl-1,4-pentadien-3-one Dibenzalacetone is a common ingredient in sunscreen. This is because dibenzalacetone absorbs UV light and helps to protect the skin from the sun’s damaging rays. The properties that are most valuable in a compound that is used in sunscreen are the compound’s abilities to absorb, reflect, or even scatter the harmful UV rays. Another importance is for the compound to not cause an allergic reaction on a person’s skin. This experiment was being performed so that dibenzalacetone could be synthesized from benzaldehyde and acetone. This experiment was performed to show how a ketone and an aldehyde could be added together through the aldol condensation. The aldol condensation is extremely important because it can form a -hydroxy aldehyde or ketone from two carbonyl compounds. This type of reaction proceeds through the creation of a resonance-stabilized enolate ion from one of the carbonyl groups. The enolate ion can then act as a strong nucleophile and add to another carbonyl group. It is extremely important that one of the carbonyl groups has an acidic alpha hydrogen (one adjacent to a carbonyl group) so that the enolate ion can be formed. Aldol products can be formed through either acidic or basic conditions and since they are usually exothermic the reaction will be driven to completion. In this experiment, you will run an aldol condensation between an aldehyde and a ketone and then the product of the reaction precipitates out of solution and can be collected by filtration. The crude product is normally purified by recrystallization. Weigh your product and determine percent yield. What reactant is your percent yield based on? Determine the melting point and compare to the literature value. (Table 1 and 2) 4 Table 1. Physical properties of compounds Compound MW Mp Bp Density Benzaldehyde 106.13 -56oC 178oC 1.04 Acetone (reagent) 58.08 Dibenzalacetone 234.30 56oC 0.79 113oC Table 2. Melting points of aldol condensation products Ketones Aldehydes Acetone Cyclopentanone Cyclohexanone 4-Methylcyclohexanone Benzaldehyde 111-113°C 189°C 118°C 98-99°C 4-Methylbenzaldehyde 175°C 235-236°C 170°C 133-135°C 4-Methoxybenzaldehyde 129-130°C 212°C 159°C 141-142°C Cinnamaldehyde 144°C 225°C 180°C 163-164°C Safety Note a) NaOH in aqueous ethanol is corrosive and particularly dangerous to the eyes. If contacted, remove with plenty of water. b) Acetone is highly flammable. c) Benzaldehyde is listed as moderately toxic (but contributes to the flavor of almonds) Experimental Procedure To 10 mL of CH3OH in a large test tube add 2.0 mL of benzaldehyde. 1.0 mL of acetone and 1.0 mL (ca. 20 drops) of 10% NaOH. Heat the solution in water bath at 50 oC for 15 minutes. Chill the solution in an ice bath and use a dropper add cold water (icy water) dropwise to precipitate the product as pale yellow crystals. Up to 1 mL (~ 20 drops) of cold water mat be added, but avoid adding excess water, which causes a milky suspension and oily product to form. Collect the product by suction filtration, wash with water, and air dry (hand dry). Determine the weight, melting point, and percent yield. Return the product to your instructor. NOTE- The amounts of the reagents used in this reaction are very important to ensure the correct product forms. In a given example a student added twice as much acetone as the procedure called for. This error on the student’s part would greatly affect the end product. Since there would be such an excess of acetone the benzaldehyde would only see acetone and would not end up adding twice to any acetone molecules. This would give an end product of benzalacetone instead of dibenzalacetone. 5 EXPERIMENT 7 (Organic Chemistry II) Aldol Condensation REPORT FORM Name _______________________________ Instructor ___________________________ Date ________________________________ Equation: _____________________________________________________________________________________ 1. Mass of benzaldehyde used (Show calculation) ________ g 2. Moles of benzaldehyde used (Show calculation) ________ mole 3. Moles of dibenzalacetone expected (theoretical yield) (Show calculation) ________ mole 4. Mass of dibenzalacetone expected (Show calculation) ________ g 5. Mass of dibenzalacetone recovered ________ g 6. Percentage yield of cyclohexene (Show calculation) ________ % 7. Structure of Dibenzalacetone 8. Literature melting point ______________ Observed melting point ________________ Range 6 Pre-Laboratory Questions–EXP 7 Name: Aldol Condensation Due before lab begins. Answer in space provided. 1. 0.791 g acetone reacted with 1.044g benzaldhyde to produce experimentally 1.00 g dibenzalacetone. Calculate the theoretical and percentage yield. 2. Using aldol or crossed aldol condensation, suggest a synthesis of the following compounds. CH2-CH3 O CH3 - CH2- CH2- CH = C - C H a) O b) C6H5 - CH = CH - C H 3. What product would you expect to obtain from aldol cyclization of hexanedioal in basic solution. O O C - CH2-CH2-CH2-CH2 - C H H 4. Write an aldol condensation product(s) between the following compounds. a) acetone + benzophenone (1:1 ratio) b) cyclohexanone and cyclopentanone (1:1 ratio) 7 Post-Laboratory Questions–EXP 7 Name: Aldol Condensation Due after completing the lab. 1. If student added two fold of acetone the acetone will react with itself and the product would be isolated as diacetone alcohol or mesityl oxide. Write a complete reaction to support your result. 2. Draw the structure of the cis and trans isomers of the compound that you prepared. Why do you imagine that? you obtained the trans isomer as the major, or even sole, product? 3. Draw a complete electronic mechanism of the aldol product between benzaldehyde and acetophenone. 4. Complete the following reactions. O base a) 2 O O b) base + H H 8