Alcohols-Carbonyl
advertisement
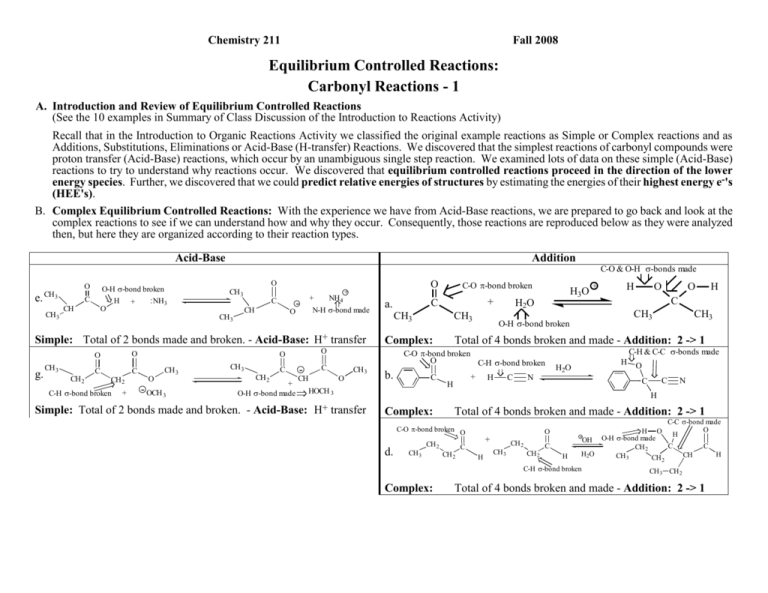
Chemistry 211 Fall 2008 Equilibrium Controlled Reactions: Carbonyl Reactions - 1 A. Introduction and Review of Equilibrium Controlled Reactions (See the 10 examples in Summary of Class Discussion of the Introduction to Reactions Activity) Recall that in the Introduction to Organic Reactions Activity we classified the original example reactions as Simple or Complex reactions and as Additions, Substitutions, Eliminations or Acid-Base (H-transfer) Reactions. We discovered that the simplest reactions of carbonyl compounds were proton transfer (Acid-Base) reactions, which occur by an unambiguous single step reaction. We examined lots of data on these simple (Acid-Base) reactions to try to understand why reactions occur. We discovered that equilibrium controlled reactions proceed in the direction of the lower energy species. Further, we discovered that we could predict relative energies of structures by estimating the energies of their highest energy e-'s (HEE's). B. Complex Equilibrium Controlled Reactions: With the experience we have from Acid-Base reactions, we are prepared to go back and look at the complex reactions to see if we can understand how and why they occur. Consequently, those reactions are reproduced below as they were analyzed then, but here they are organized according to their reaction types. A Acid-Base O e. CH3 CH 3 O-H -bond broken .. NH H + 3 O C CH Addition O O CH 3 C CH CH 3 O + - NH4 + N-H -bond made Simple: Total of 2 bonds made and broken. - Acid-Base: H+ transfer O O g. CH 3 CH 2 C C CH 2 C-H -bond broken + CH 3 - OCH 3 CH 3 CH 2 C + O-H -bond made - C CH O CH 3 CH3 CH3 H3O H2O b. C + H O O H C CH3 O-H -bond broken CH3 Total of 4 bonds broken and made - Addition: 2 -> 1 C-O -bond broken O C-H -bond broken HOCH 3 Simple: Total of 2 bonds made and broken. - Acid-Base: H+ transfer + C Complex: O O O a. C-O -bond broken C-O & O-H -bonds made + H H C N C-H & C-C -bonds made H H2O O C C N H Complex: Total of 4 bonds broken and made - Addition: 2 -> 1 C-O -bond broken O d. CH 3 CH 2 CH 2 O + C H CH 3 CH 2 CH 2 C H C-C -bond made O H O H OH O-H -bond made C C CH 2 H2O H CH CH 3 CH C-H -bond broken Complex: 2 CH 3 CH 2 Total of 4 bonds broken and made - Addition: 2 -> 1 Reactions of Carbonyl Compounds-1 c. C-O - and -bonds broken 2 N-H -bonds broken O CH 3 CH 3 Complex: f. O C Complex: C .NH2OH + CH 3 Substitutions C-N - and -bonds made O N H2O pH ~5 CH 3 C CH 3 O H 2 O-H -bonds made CH 3 + C-N -bond broken NH2 + + H3O 2 O-H -bonds broken C H2O Total of 8 bonds broken and made - Substitution: 2 -> 2 O C 2 h. C-H -bond broken Complex: C-O -bond made O H + 2 N-H -bonds made i. C CH3 Cl O O C - OCH 3 H C CH 3 O O-H -bond made + HOCH 3 C-C -bond made Total of 4 bonds broken and made - Substitution: 2 -> 2 O O C-Cl -bond broken + NH4 C-O -bond broken CH 3 O O H H C CH 3 O + C 2 CH3CH2OH O-H -bond broken CH3 + C-O -bond made - CH2CH3 + Cl O + CH3OH2 O-H -bond made Total of 4 bonds broken and made - Substitution: 2 -> 2 Complex: C-O - and -bonds broken j. CH3 CH2 Complex: 2 N-H -bonds broken NH2 O HN C + H CH3 H2O HN Total of 4 bonds broken and made - Substitution: 2 -> 2 C H2O + NH3 C-N - and -bonds made CH2 N N H 2 O-H -bonds made H Total of 8 bonds broken and made - Substitution: 2 -> 2 C. Correlation of the Structures of Carbonyl Compounds with their Reactivity: Look at the patterns of reactivity for each reaction type of the carbonyl compounds summarized in Section B and try to develop some specific correlations between the structures of reactants and the outcome of the reactions. Specifically: All reactions except acid-base reactions seem to involve the carbonyl carbon and/or oxygen. a. Examine the specific bond changes occurring for each type of reaction. Can either of the complex reaction types (Addition or Substitution) groups be subdivided? Explain. Substitution can be subdivided more because if we look at the complex substitution (c) with 8 bonds, it has a π-bond and the bond can be subdivided to a single bond. It can be subdivided to 4 bonds. Then (h) can be subdivided to σ bond. 3 Reactions of Carbonyl Compounds-1 b. Look more closely at how the reaction outcome (addition or substitution) relates to the overall structure of the carbonyl compound in the reactants. Is there any correlation between the carbonyl compound structure in the reactants and the overall reaction that takes place? Explain. The bonds get yield for the heteroatom. Aldehydes can be found in the addition and substitution. c. Is there any correlation of the structure of the other reactant (not considered in b. above) and the overall reaction that takes place? Explain. Reactions of Carbonyl Compounds-1 4 D. Classification of Reactions According to : 1. Definition of Reaction Mechanism: The REACTION MECHANISM is the sequence of reaction steps by which the structures of the reactants are rearranged as the reactants proceed to the products. In the following reaction, there are several bonds rearranged and it is not immediately obvious as to how this could occur in one reaction step. + Br 2 H2O + H3O O + Br H Products Reactants 2. Tools of the Trade: Curved Arrows As we will see in CHEM 212, the above relatively complex reaction can be dissected into the series of simple reaction steps shown below, which can be represented by curved arrows as indicated. a Br + 2 H2O Reactants b H2O c Br H2O Intermediate #1 d H H2O O H Br Intermediate #2 O H H3 O Br Products As we discovered in Acid-Base-7, the arrows represent the movement of . In addition to aiding in drawing new resonance structures, curved arrows can also help in predicting and understanding the steps in complex reactions. However, in illustrating reaction steps, arrows depict a bit wider range of electron pair movements. a. What changes in electron positions are illustrated by arrow a above? How many electrons were moved? Electrons are moved from a bond between bromine and carbon- 2 electrons are moved. b. What changes in electron positions are illustrated by arrow b above? How many electrons were moved? Carbon and oxygen are made- two electrons are made. c. What changes in electron positions are illustrated by arrows c & d above? How many electrons were moved with each arrow? An oxygen loses a lone pair of electrons which makes a proton -2 electrons are moved d. How are these electron movements different from those we encountered in using curved arrows to draw resonance structures in Acid-Base-7. Be as specific as you can. Pi bonds involved; arrows between molecules. 5 Reactions of Carbonyl Compounds-1 2. Applications of Curved Arrows to illustrate reaction steps: a. Draw curved arrows to account for the movement of electrons in each of the following one-step reactions and classify each reaction as either substitution, addition or elimination: 1. H .. C N .. .O. H 2. + CH3 H .. .I. H H3N CH3 + H H .. .I. .O. .. H elimination H C H substitution .. .O. .. .. C + .O. .. .. .. HO H 3. .. HO .. + C .. .. NH3 H N .. .. H .. H H addition b. Directions of the arrows: 1. For the following reaction, draw the structure of the product formed by the electron movement illustrated by the curved arrow. Be sure to include all lone pairs of electrons. .. .. HO .. H C + .O. .. H 2. The reactants here are the same as those in 2.a.3. above. How is your product here similar to the product provided in reaction 2.a.3. above? 3. How are the two products different? 4. Based on the energies of the HEE, which product is more likely to form? Explain. 5. What do the above results suggest about the most favorable direction of electron movement in reactions? Reactions of Carbonyl Compounds-1 6 E. Mechanistic Analysis of Complex Reactions of Carbonyl Compounds (See section B.): Evaluating Potential Reaction Mechanisms: For the following reaction, select the mechanism (the sequence of steps) that you believe to be most reasonable. Remember that the most likely mechanism is generally the one in which the e- energies are kept the lowest throughout the sequence of steps. One high energy intermediate will make the mechanism less likely. So base your choice on the energies of the e-'s of the intermediates as the reaction proceeds. Explain the logic of your choice. Mechanism A + : OH + - : OH - + : : - :O : : : O H : : - : : H O+ H H : OH : : + : :O: : OH : : : Mechanism B HEE moves in each step. Starts in OH and it moves to oxygen atom. Then on the next step the HEE is on oxygen. H : : : OH :O: O :O : + OH H H + -: OH + H O O 2 : : : : : : : : Mechanism C : OH : - : : + O+ H H + - : : :O : H : OH : : H2O Out of Class Applications: A. Reading Assignment: CGWW pp. 123-133 B. Problems: CGWW pp. 3 & 5 :O : + - : : : - :O: : OH 7 Reactions of Carbonyl Compounds-1 Using "Curved Arrows" to describe Organic Reactions C. For each of the following reactions, write structural formulas for the products that would be formed if the transformation indicated by the "curved arrows" were to occur. (Be sure to include both the bond sequences and the charges on atoms in the products.) Then classify the overall reaction as an addition, elimination, substitution or acid-base reaction. 1. O N + Cl 2. CH 3 O - CH 3 + CH 2 + - O CH 3 + CH 3 CH 2 Cl H CH 3 Br 3. + O H CH 3 C C 4. O CH 3 Cl C O Br H - D. Classify each of the following reactions as an addition, elimination, substitution or acid-base reaction. Then, draw the "curved arrows" that would account for the transformation in one step. Br Br + 1. .. C CH3 NH2 C CH3 NH2 + O O 2. Br - + + Br 3. C CH 2 4. + I + H + C Cl .. NH3 + I - + CH 3 + Cl .. NH3 I - + + NH4 Classify each step separately and then classify the net effect of both steps together. Reactions of Carbonyl Compounds-1 8 Investigating Reactions of Carbonyl Compounds E. Using the structure-reaction correlations discovered in this activity, predict structures for the most likely products of the reactions listed below. No mechanisms are needed here. Explain the logic that led to your predictions. O 1. CH3 2. CH3 C O O C O CH2 CH2 3. + H H C N O H2 O C CH2 O - OCH2 CH3 + CH3 C CH2 CH3 CH2 CH3 O O + CH2 H O 4. - OH C NH2 H H2 O H2O + pH = 6 F. For the following reaction, select the mechanism (the sequence of steps) that you believe to be most reasonable. Remember that the most likely mechanism is generally the one in which the e- energies are kept the lowest throughout the sequence of steps. One high energy intermediate will make the mechanism less likely. So base your choice on the energies of the e-'s of the intermediates as the reaction proceeds. Explain the logic of your choice. :O : :O : :O : : : + : OH : : O: + H2O : : O :O : - : : : : - O H Mechanism 1: :O : - :O : : :O: : : H2O O: + H2O Mechanism 2: OH H :O : - O: + :O : : : + H2O O : : : : O Solvent molecule regenerated : : Mechanism 3: - + : OH : : Solvent molecule used O :O : - : O : : : + : OH : : - : : : : O H2O H O+ H : :O : - : O : : :O: : : :O : :O : : : O - : : + : OH :O : OH : : : : : : : : O - : : :O : O H 9 :O: :O : H2O - O: + :O : : : + : OH : : - :O : - : : O: + : : + : OH H2O : : - : : : : O : : :O : Reactions of Carbonyl Compounds-1 : O: O H G. Nomenclature of Aldehydes and Ketones References: 1. CGWW: Ch. 2 p. 38-9 2. Tutorials: a. http://chemistry.boisestate.edu/rbanks/organic/nomenclature/organicnomenclature1.htm Developed by Richard C. Banks, Professor of Chemistry, Boise State University Provides questions with answers Sections Aldehydes Ketones b. http://www.molecularmodels.ca/nomenclature/index-2.htm Developed by Professor Dave Woodcock, Okanagan University College, British Columbia, Canada (Contains many examples.) Sections: 5. Functional Groups with Suffix and Prefix IV. Alkanones (Ketones) V. Alkanals (Aldehydes) Note: Your browser must have the chemscape chime plug-in for these pages to work. Select "1. Introduction to these pages", then click on "Nomenclature Index - Chemscape Chime" and begin with the "How and Why". If you have problems, go to the http://www.molecularmodels.ca/nomenclature/nom1.htm and click on "chemscape chime" and follow directions to download the "chemscape chime plug-in." If you have problems contact me. 3. http://www.acdlabs.com/iupac/nomenclature Developed by Advanced Chemistry Development Laboratories (Gives detailed IUPAC rules for nomenclature.) Recommendations 1993 R-5 Applications to Specific Classes of Compounds R-5.6 Aldehydes, Ketones, their Derivatives and Analogues Reactions of Carbonyl Compounds-1 R-5.6.1 Aldehydes, thioaldehydes, and their analogues R-5.6.2 Ketones, thioketones, and their analogues. 10 4. Applications 11 Reactions of Carbonyl Compounds-1 a. Name the following: O O O O O b. Draw structural formulas for the following compounds: 3-phenyl-2-pentanone 4-bromo-3-ethyl-5-heptenal 3-cyclopentenone 4-methoxybenzaldehyde







