Anemia
advertisement

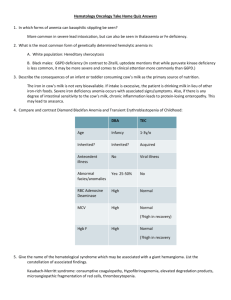
Heinz Bodies – G6PD deficiency, Thalassemia, unstable hemoglobinopathies Bite cells, Blister cells – G6PD deficiency Schistocytes – MAHA, DIC, TTP Spherocytes – hereditary spherocytosis, AI warm-Ab hemolytic anemia Target cell—Thalassemia, liver disease, HgbC, after splenectomy Howell-Jolly bodies – post-splenectomy Petechial rash – thrombocytopenia Large platelets in marrow – ITP, peripheral destruction Ringed sideroblasts – myelodysplastic syndromes Hypersegmented PMNs – megaloblastic ds – B12/folate deficiency Teardrops - myelofibrosis Deficiency Anemia Dx: MCV and reticulocyte count (normally 1% of circulating blood cells) – with anemia, ret count should increase if bone marrow is working properly - if rets are low and MCV is low microcytic anemia Fe deficiency or thalassemia - if rets are low and MCV is high macrocytic anemia B12/folate deficiency - if rets are high blood loss, hemolysis Tx: 1. determine cause of anemia and correct underlying cause 2. transfuse in emergent situation (angina pectoris, shock, surgery) - make decision based on time – if tolerating anemia well, don’t transfuse - unit of RBCs (250ml) will increase Hb by 1gm and hematocrit by 3% - risks of transfusion (HBV > HCV > HIV) Iron deficiency anemia: Causes: Inadequate intake (children – still building Fe pool) Diversion of iron during pregnancy (adult females) Blood loss (adult males and females) Dx: 1. Serum ferritin – stored in RE system, but a little circulates – low levels deficiency 2. Bone Marrow – stain marrow for iron 3. Treatment – put them on Fe and if it fixes problem, pt is iron deficient Megaloblastic Anemia/Folate deficiency anemia: Causes: Dietary Malabsorption (from the upper small bowel) Increased usage (pregnancy) Dx: Morphology (macrocytic RBCs) Serum folate (rarely measured) RBC folate (rarely measured) Tx: folate supplement – 1gm/day B12 Deficiency Anemia: Causes: Pernicious Anemia – Ab to parietal cells – don’t make IF, can’t absorb B12 Pancreatic insufficiency – not enough secretions to get B12 and IF complexed Malabsorption - problem with terminal ileum – chronic inflam. bowel ds Dx: morphology – identical to folate def. – macrocytic, hypersegmented PMNs Serum B12 – good test Neurologic findings – demyelination of the spinal cord – tabes dorsalis Tx: B12 supplementation IM B12 - lifelong Hemolytic Anemia diagnostic of Fe Clinical features: splenomegaly, pigmented gallstones (↑ bilirubin), dark urine, chronic ankle ulcers, aplastic crises (associated with parvovirus B19), ↑ folate requirement Laboratory features: 1. Increased formation of RBCs Blood: ↑ rets, ↑ MCV, circulating NRBCs Bone marrow: erythroid hyperplasia, reduced M:E ration (more erythroid than myeloid) Bone: increased marrow production frontal bossing 2. Increased destruction of RBCs – general hemolysis Biochemical: ↑ LDH levels, ↑ unconjugated bilirubin Physical: scleral icterus, jaundice Intravascular hemolysis: Reduced serum haptoglobin Hemoglobinemia Hemoglobinuria Hemosiderinuria Hereditary Spherocytosis – membrane defect - most common defect leading to anemia, AD - membrane defect in spectrin or ankyrin - Spherocytes – bits of membrane are pinched off by spleen lack central pallor, more spherical due to loss of membrane Dx: ↑ osmotic fragility Tx: folate supplementation Splenectomy – spherocytes will persist, but hemolysis will abate G6PD Deficiency – enzyme defect - deficiency is X-linked – enzyme variant A- is unstable and has low activity - 10-14% of African-Americans have this disorder, Mediterraneans also have low-activity variant - agents to avoid: anti-malarials, sulfa, dapsone, Vit K, naptha compounds, fava beans Pathophysiology: • Low G6PD activity results in low levels of NADPH and reduced glutathione, which are required to protect hemoglobin from oxidative damage. • In the absence of adequate reducing ability (provided by G6PD), oxidizing agents convert hemoglobin to methemoglobin, then denature it, causing it to precipitate as Heinz bodies. • The spleen pinches off the Heinz body and the overlying membrane, leaving a “bite cell” or “blister cell” Clinical features: - hemolysis triggered by drugs or infection - anemia is maximal 7-10d after exposure - A- individuals compensate with ↑ ret formation - immediate after hemolytic episode, G6PD levels may be normal because only the newly formed cells are present Immune-mediated hemolytic anemia – acquired hemolytic anemia Pathophysiology: • Red cells react with antibody, with or without complement fixation, leading to RBC destruction. • IgG-coated RBCs interact with Fc receptors on macrophages, leading to complete or partial phagocytosis, with spherocyte formation (extravascular hemolysis). • C3-coated red cells interact with C3 receptors on macrophages, with phagocytosis (extravascular hemolysis). • Alternatively, complement cascade can be completed, with complement-mediated RBC lysis (intravascular hemolysis). Auto-immune hemolytic anemias: 1. 2. - Warm-Ab react with RBCs best at 37 and typically do not agglutinate red cells Cold-Ab react best at <32 and typically DO cause agglutination Coomb’s test – looks for Ab (IgG or C3, NOT IgM) to RBCs which are either bound to red cells or floating in the circulation – positive test is defined as clumping in the test tube Direct Coomb’s test (DAT) – looks for IgG or C3 directly bound to red cell surface Indirect Coomb’s test – looks for IgG in the serum which are capable of reacting with normal red cells Warm-Ab Hemolytic Anemias (AIHA – Autoimmune hemolytic anemia) Sx: fatigue, shortness of breath or chest pain, “tea-colored urine”, pallor, jaundice, splenomegaly Dx: increased rets, increased bilirubin, and increased LDH Positive Coomb’s test (both direct and indirect) Spherocytes on peripheral smear Associated with platelet destruction – Evan’s Syndrome Tx: immunosuppression – allow pt’s bone marrow to regenerate RBCs 1. corticosteroids 2. splenectomy 3. stronger immunosuppression – cyclophosphamide or azathioprine folate repletion Drug-induced hemolytic anemias: 1. Innocent bystander – Ab develop against drug, drug and Ab bind together to form immune complexes, deposit on surface of RBC where they are recognized by RE system – drug must be present for hemolysis to occur – occurs with quinine, quinidine, isoniazide 2. Hapten mechanism – drug binds to RBC surface, Ab form which are directed against complex of RBC/drug, hemolysis – drugs: penicillin and cephalosporin 3. True autoimmune mechanism – certain drugs induce formation of Ab directed against RBC surface components – drugs: Alpha-CH3-DOPA, L-DOPA, procainamide Cold-Agglutinin Disease Pathophysiology: • Pathogenic antibodies are usually IgM, which bind to red cells in the cooler extremities, then fix complement. When red cells return to the warmer torso, IgM falls off. • Complement-coated red cells can be lysed directly within the vessel (intravascular hemolysis). • Alternatively, complement-coated red cells can be engulfed by complement receptors on macrophages within the liver (extravascular hemolysis) Clinical features: • Can be associated with infection with either Mycoplasma or Mononucleosis, • Can also be idiopathic or associated with a lymphoproliferative disease. • Episodic, cold-induced hemolytic episodes – cold-induced cyanosis in fingers, toes, nose and lips Tx: • Treatment is to keep patient (especially the extremities) warm. Blood and IV fluids should be warmed. • Steroids and splenectomy are usually ineffective. • Immunosuppression with oral chemotherapy may be required. Non-immune hemolytic anemia (MAHA) • Shear damage to red cells as the result of endothelial cell activation/damage. • Hallmark is presence of schistocytes on the peripheral smear. • Can be caused by the following disorders: – TTP/HUS – DIC – Malignant hypertension – – – Ecclampsia, HELLP syndrome Vasculitis Other, including metastatic cancer, scleroderma renal crisis, solid organ transplant rejection Hemoglobinopathies Definition: caused by a mutation resulting in an amino acid substitution or deletion on one of the globin chains Effects: no detectable effect, instability of hemoglobin molecule, increase or decrease in O2 affinity, inability to maintain the heme iron is its active, reduced state (methemoglobinemia), decreased solubility of the hemoglobin molecule α – globin genes on Chromosome 16, non- α – globin genes on Chromosome 11 Types: 1. Unstable hemoglobinopathy: H2O able to reach heme pocket, leading to denaturation of heme and formation of Heinz bodies 2. Alteration in Hb-O2 affinity: mutation stabilizes either the oxy or deoxy conformation - increase in O2 affinity – left-shift in O2 saturation curve – individuals exhibit erythrocytosis (increased RBCs - need increased O2 carrying) – ex. Hb Bethesda - decrease in O2 affinity – right shift in O2 saturation curve – individuals typically somewhat anemic (decreased RBCs) 3. Inability to maintain heme iron in active, reduced state: methemoglobinemia – HbM disorders occur when there is a substitution at locus of proximal or distal histidine – Hb with iron in oxidized (Fe3+) state is incapable of binding O2 – patients with HbM disease are typically cyanotic 4. Decreased solubility of hemoglobin molecule: Sickle cell anemia is autosomal co-dominant mutation - At the 6-position of the Beta-chain of hemoglobin, you have a gluval substitution, which leads to polymerization of the Hb molecule – sickle-cell trait = roughly equal amounts of HbA and HbS, asymptomatic – compound heterozygotes express significant disease – presence of HbS decreases solubility and tendency of this abnormal Hb to polymerize when it is in the deoxy conformation – allows Hb to form polymers – Clinical features – affects virtually every organ in body - Associated with gallstones, nonhealing leg ulcers (probably b/c of vaso-occulusion), “acute chest syndrome” which increases morbidity and mortality, heart problems (dilation, hypertrophy), pulmonary htn – Tx: reactivate fetal Hb products with hydroxyurea – HbF increases the solubility in deoxy form Thalassemia - characterized by imbalance in the production of a-globin and non a-globin chains - excess, unpaired globin tetramers become denatured and precipitate within red cells forming Heinz bodies or target cells - small, poorly hemoglobinized red cells (microcytosis and hypochromia) Coagulation Defects Hemophilia A - 85% of Hemophilias X-linked disease—mostly boys affected classified Severe (<1% of normal factor VIII levels), Moderate (2-5%), Mild (6-30%). Factor VIII deficient o Produced in the liver, along with the other coagulation factors o Not a protease itself, but rather a cofactor that helps to accelerate the coagulation reaction. o APTT will be prolonged. Factor VIII is part of the intrinsic pathway. Target joints—bleeds into specific joints o Symptoms usually precede evidence of bleeding o Can use radio-synovectomy to get rid of synovium and minimize bleeding Can use factor as prophylaxis in severe cases—can administer via Port-A Cath Factor VIII replacement now is recombinant product, with only human albumin used to stabilize the factor. In the past, human factor was used. This resulted in 80-90% of hemophilia patients getting HIV. Vaccinate patients for HepA This disease is a good candidate for Gene Therapy! Hemophilia B a deficiency of factor IX. About 15% of hemophilias Good candidate for gene therapy—smaller gene than factor VIII For both, potential for formation of antibodies against factor (aka “inhibitor”) o Factor recognized as “non-self” and B-cells form antibodies that get rid of it o In normal individuals (ie, those without hemophilia), treat with rituxan, which latches on to CD20+ B-cells and neutralizes them. o In hemophiliacs, try to de-sensitize by giving MASSIVE infusions twice daily. Generally in little kids Must be religiously adhered to, or the immunity is reactivated von Willebrand Disease (VWD) Differs from “classical hemophilia”: vWF important in both primary hemostasis (platelet adhesion via GPIb receptor) and secondary hemostasis (via Factor VIII) (1) Suffer from mucocutaneous hemorrhage rather than hemarthroses like in hemophilia. (2) Autosomal inheritance trait, so men and women have similar prevalence, rather than X-linked like hemophilia. (3) Consistently had prolonged bleeding times unlike the normal bleeding times in hemophilia. Also have prolonged APTT (involvement of Factor XIII) Thrombocytopenia • Symptoms of platelet hypofunction - epistaxis, gum bleeding, bruising, heavy menses, petechiae, i.e. mucocutaneous bleeding. “Oozing and bruising” NOT hematomas or bleeding into joints. Three causes: 1. underproduction – inadequate megakaryocytes in the marrow due to failure, invasion, injury (especially EtOH), congenital 2. peripheral destruction – DIC, TTP, ITP - non-immune mechanisms, immune mechanisms (drugs, HIV) 3. splenic sequestration - Splenic enlargement, from any cause, can lead to displacement of platelets from peripheral circulation into splenic pool DIC Definition: A process, characterized by abnormal activation of coagulation, generation of thrombin, consumption of clotting factors, destruction of platelets, and activation of fibrinolysis. Dx: • Elevated PT first - due to consumption of Factor VII, which has the shortest half-life (4 hrs) of all clotting factors. • Coagulation tests are elevated • When advanced, the APTT can be prolonged as well, as the other clotting factor levels fall. • Low platelets • Low/falling fibrinogen • Elevated fibrin degradation products (FDPs/FSPs) or D-Dimers • Can see a few schistocytes on the peripheral smear in most cases. Tx: Can be associated with gram negative sepsis, severe burns, obstetrical disasters, certain leukemias or tumors, shock, insect or snake venoms • • TREAT THE UNDERLYING CAUSE Supportive measures can include transfusion of platelets, clotting factors, fibrinogen, +/- low dose heparin to halt thrombin generation. TTP – Thrombotic Thrombocytopenic Purpura Definition: A process, characterized by abnormal activation of platelets and endothelial cells, with fibrin deposition in the microvasculature, and peripheral destruction of platelets and red cells. Causes: • May be associated with an antibody against or a deficiency of the protease which cleaves the very high molecular weight multimers of von Willebrand’s factor • Can be induced by drugs, including ticlopidine, quinine, cyclosporine, FK-506, mitomycin C • Increased incidence with pregnancy or HIV Dx: MAHA, low platelets, schistocytes • Microangiopathic Hemolytic Anemia (MAHA) – Elevated LDH, elevated bilirubin – Schistocytes on the peripheral smear – MUST BE PRESENT • Low platelets - MUST BE PRESENT • Fever – not always seen • Neurologic Manifestations – not always seen - headache, sleepiness, confusion, stupor, stroke, coma, seizures • Renal Manifestations – not always seen – hematuria, proteinuria, BUN/Creatinine Tx: Plasma Exchange • Treatment relies on PLASMA EXCHANGE. • DO NOT GIVE PLATELETS • Remove all inciting agents. • Adjunct therapies, including glucocorticoids and anti-platelet agents can be used but are of uncertain benefit. • Secondary measures if no response to plasma exchange include splenectomy, vincristine. -sometimes associated with HUS (hemolytic uremic syndrome) – related to diarrheal diseases, esp in children Thrombocytopenia – Drug/Immune Mechanism • Drugs can lead to immune-mediated thrombocytopenia by a variety of mechanisms. 1) directly stimulating anti-platelet antibody production 2) a hapten mechanism 3) “innocent bystander” phenomenon. - Drugs to avoid: • Beta-lactam antibiotics • Trimethoprim-sulfamethoxazole and other sulfa drugs • Quinine/quinidine • Heparin • Abciximab (ReoPro®) • Heparin induced thrombocytopenia ITP – Immune/Idiopathic Thrombocytopenic Purpura • Diagnosis of exclusion - there is no test • Need to exclude splenomegaly and occult thyroid disease. • Megakaryocytes should be present, large platelets • In children, usually provoked by viral illness. – Spontaneously remits – No specific therapy usually required. • In adults, specific therapy required if platelet count is <50 or if patient is bleeding. Tx: steroids, IVIg, WinRho if Rh+ • First line – steroids and IVIg • Initial therapy relies on use of corticosteroids (e.g. prednisone). These can take 48-72 hrs to take effect. • If platelet count is <10K or if patient is actively bleeding, need more rapid therapy--use IVIg • If patient is Rh positive, can use Anti-D (WinRho®) in place of IVIg. Body goes after red cells instead of platelets • Second-line tx is splenectomy • If pt relapses, stronger immunosuppression is required - cyclophosphamide, azathioprine, cyclosporine Thrombocytosis/Thrombocythemia • Abnormal platelet activation results in arterial occlusion--if in the CNS, these are strokes. If in the coronary arteries, these are heart attacks, and if in the peripheral arteries, these become gangrenous limbs Dx: Platelet function screen – prolonged bleeding time (APTT, PT, TCT will be normal) Leukocytes Disorders Neutrophilia- acute infections, acute inflammation, myeloproliferative ds (esp CML), G-CSF, corticosteroids Neutropenia- anti-psychotics, anti-epileptics, anti-thyroid, some abx, immune (SLE, RA) Agranulocytosis – almost always drug induced – clozaril, propylthiouracil, anti-convulsants, chloramphenicol and sulfa abx EosinophiliaN – neoplasm A – asthma/allergy A – Addison’s Ds C – collagen vascular ds P – parasites Basophilia – myeloproliferative ds Lymphocytosis – viral infection, chronic lymphocytic leukemia (CLL) Lymphocytopenia – immunosuppression, immunodeficiencies (HIV/AIDS), lymphomas, granulomatous ds (sarcoidosis, TB) Monocytosis – bacterial infxn, protozoal infxn Acquired defects in neutrophil function: Alcohol Chronic corticosteroid use Leukemia Myeloproliferative ds Myelodysplasias Inherited Neutrophil Abnormalities: Chediak-Higashi Chronic Granulomatous Ds MPO Deficiency Leukocyte Adhesion Deficiency Myeloproliferative Disorders – Primary Erythrocytosis Definition: Myeloproliferative disorders are Stem Cell Disorders leading to autonomous production of hematopoietic cells from ALL THREE LINEAGES (red cells, white cells, platelets). Polycythemia vera Definition: A neoplastic disorder arising from a pluripotent stem cell, generally characterized by erythrocytosis, with or without concomitant thrombocytosis and leukocytosis. In P. vera, many if not all of the circulating blood cells are derived from a single neoplastic stem cell. The erythroid progenitor cells of patients with P. vera, are capable of growing and dividing in the absence of erythropoietin Dx: no definitive diagnostic test PE: splenomegaly, hepatomegaly, facial plethora Blood smear: thrombocytosis, basophilia and neutrophilia Lab: low or undetectable EPO levels, high LAP (CML is low), increased uric acid and B12 Sx: Pruritis after bathing Erythromelalgia (burning sensation in palms and soles) Hypermetabolic symptoms – fever, weight loss, chills Thrombosis in an artery or vein (esp hepatic vein Budd-Chiari) GI bleeding Tx: Phlebotomy, myelosupression (hydoxyurea, 32P, busulfan), IFN-a Essential Thrombocythemia Polyclonal increase in megakaryocytes Dx: rule out secondary causes of thrombocytosis (cancer, infection, inflammation, bleeding), splenomegaly, platelets >600 on two separate occasions Sx: Microvascular thrombi—digital ischemia Erythromelalgia and pruritis Hemorrhagic symptoms—primary platelet dysfunction, and also, platelets over 1 million eat up vWF and cause deficiency splenomegaly Lab findings: Iron studies normal Coagulation studies normal Pseudohyperkalemia and psuedohypoglycemia (platelets use sugar and secrete K+) Bizarre platelet morphology Marrow shows abnormal megakaryocytes Tx: Treat when: over 65, had thrombotic event, count over 1 million Treat with: anagrelide – interferes with megakaryocyte maturation hydroxyurea IF-alpha Myelofibrosis Definition: Clonal stem cell disorder affecting megakaryocytes predominantly. Can be secondary to any of the myeloproliferative ds Sx: anemia, thrombocytopenia, fever, sweats, weight loss, massive splenomegaly, hepatomegaly Dx: Peripheral smear shows leukoerythroblastic picture, with teardrops, NRBC and early granulocytes. Bone marrow aspiration often gives “dry tap” Tx: No definitive therapy. Treatment is supportive, with transfusions. Splenectomy to relieve abdo pain Myelodysplastic Syndromes Definition: Conditions in which there is disordered maturation in 1 or more cell lines, usually producing cytopenias. Usually found in the elderly Dx: abnormal CBC, macrocytosis of RBC, large platelets, PMNs hypogranular or bilobed nucleus Bone marrow: megaloblastic erythropoiesis, ringed sideroblasts, small megakaryocytes Monosomy 5, 7, 8 can be seen