Unit One – What is Chemistry
advertisement

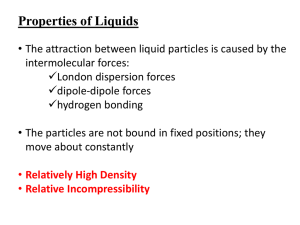
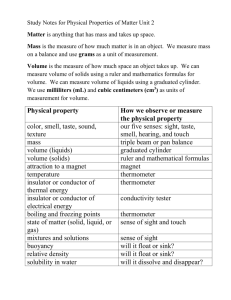
Page 1 Unit One – What is Chemistry Sections 16.1-16.3 16.1- Chemistry: The Central Science Write the definition of chemistry as given in class: 16.2-The Submicroscopic World Atom – the smallest component of an element having the chemical properties of the element Molecules – two or more atoms chemically bonded or combined together. Can be the same or different types of atoms. Examples: Macroscopic – can be seen without magnification. It can be measured and handled Microscope – need an instrument to been seen States of matter – solids, liquids, gases and plasma 1. Solids a. have a definite shape b. have a definite volume c. the particles are packed closely together Page 2 d. types of solids 1) crystalline solids - have a regular, repeating pattern of particles - examples: salt, diamond 2) amorphous solids - particles do not repeat in a pattern - these solids do not retain their solid shape permanently - examples: wax, glass 2. Liquids a. has no definite shape b. has a definite volume c. have viscosity: the resistance of a liquid to flow - higher viscosity- less flow, lower viscosity – easier flow 3. Gases a. has no definite shape b. has no definite volume c. the particles tend to spread out more than in solids, liquids d. Boyle’s Law – if the volume of a gas is decreased, the amount of pressure the gas exerts increases (and vice versa) Page 3 e. Charles’s Law – as the temperature of a gas increases, the volume of the gas increases (and vice versa) 4. Plasma - rare on earth, but very common in stars - very high energy phase of matter - present in fluorescent light bulbs when they are on and lightning Draw the particles in a solid, liquid, and gas in the boxes below. Make sure to label which box corresponds to which phase. *****In your own words paraphrase what Ms. D said about the difference the molecular view in solids liquids and gases. Pay particular attention to solids and liquids. 16.3 –Change of Phase melting – change from solid to liquid melting point – temperature & pressure at which a solid changes to a liquid - most substances have a characteristic melting point - example: ice -> water at 0 degrees C freezing – removing heat energy to change a liquid into a solid Page 4 freezing point – temperature at which a liquid changes into a solid vaporization – the change of a substance from a liquid to a gas evaporation – a type of vaporization in which the particles of the liquid change into a gas at the surface of the liquid boiling - – a type of vaporization in which the particles of the liquid change into a gas inside the liquid and then travel to the liquid’s surface and into the air boiling point – the temperature & pressure at which a liquid boils - most substances have a characteristic boiling point - example: water -> water vapor at 100 degrees C - if air pressure is lower, boiling point is reduced (boiling point of water lowers below 100 degrees C) - if air pressure increases, boiling point is increased (boiling point of water raises above 100 degrees C) condensation – gas changes to a liquid as it loses heat energy - example: dew sublimation – surface particles of a solid change into a gas without passing through a liquid phase example: carbon dioxide, drying wet clothes outside in winter Phase Changes – Matter changes its state due to changes in its energy (heat) content - All phase changes are physical changes (the material is not changed) Energy and Phase Change Diagram Page 5 -Energy is measured in joules -Heat of Fusion: the amount of energy needed to change any substance from solid to liquid (and vice versa) -each substance has their own particular heat of fusion value -water = 334 J/g -Heat of vaporization: the amount of energy required to change any substance from liquid to gas (and vice versa) - each substance has their own particular heat of fusion value - water = 2265 J/g Why is heat of vaporization higher than heat of fusion? Can you add heat to ice without melting it? Yes, adding energy lowers the temperature even further below 0 ˚C 16.4 Physical and Chemical Properties Physical property – any property of matter that can be measured or observed without changing its chemical nature Two types a. Extensive – depends upon the amount of matter present Page 6 Ex. volume, mass, length b. Intensive – does not depend on the amount of matter present. Ex. melting and boiling point, color, density Physical change – a change that affects any physical properties Chemical property – a property of matter that can be observed only when substances interact with on another. Chemical properties characterize the ability of a stance to react with other substances or to transform from one substance to another Example: helium is very unreactive and sodium reacts violently with water Chemical change – a change that produces one or more new substances ***During a chemical change, there is a change in the way the atoms are chemically bonded to one another. Chemical Bond – the force of attraction between two atoms that holds them together. Chemical change means the same thing as chemical reaction Chemical reaction (rxn) – new materials are formed by a change in the way atoms are bonded together. Page 7 16.5 – Determining Physical and Chemical Changes **After a physical change, the molecules are the same as the ones you started with. After a chemical change, the original molecules have been destroyed and new ones are in their place. Indications of a chemical change 1. The evolution of a gas (HCl and Mg) 2. The formation of a precipitate (solid) (AgNO3 and HCl) 3. The evolution or absorption of heat 4. The emission of light (Mg and O2) 5. A color change in the reaction system (acid and base) Page 8