Management of Extrvasations
advertisement

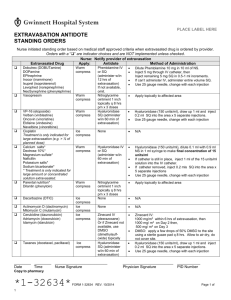
Draft Title: IV Medication, Extravasation Management Distribution: Hospital Wide Desired Outcome: 1. Patients receiving IV medication with the potential for causing irritation/tissue necrosis will be monitored closely to prevent extravasation. 2. If infiltration of a high risk drug occurs, extravasation management will be implemented to minimize tissue damage. 3. Patient will be instructed on measures to prevent extravasation, symptoms, interventions and appropriate follow-up management if extravasation occurs. Definitions: Extravasation: Passage or escape into tissue of antineoplastic drugs. Tissue slough and necrosis may occur if the condition is severe. Vesicant: An agent that has the potential to cause blistering, severe tissue injury or tissue necrosis when extravasated. Irritant: Any agent that causes aching, tightness, and phlebitis along the vein or at the injection site, with or without a local inflammatory reaction but does not cause tissue necrosis. Flare Reaction: A local allergic reaction to an agent, manifested by streaking or red blotches along the vein, but without pain. The most damaging, VESICANT EXTRAVASATION, results when chemotherapeutic agents that are capable of causing tissue necrosis infiltrate from vein to subcutaneous tissue and cause progressive, severe tissue damage. The degree of damage is related to the amount of drug that has infiltrated and the vesicant properties of the drug. A list of recommended antidotes and topical treatments for specific drugs is located on the SJMC intranet under the pharmacy section. A list of antineoplastic drugs that are classified as vesicants and irritants include the following commercially available agents: VESICANTS Cisplatin (in concentrations of 0.5mg/ml), Dactinomycin, Daunorubicin, Doxorubicin Epirubicin, Esorubicin, Idarubicin, Mechlorethamine hydrochloride, Mitomycin, Mitoxantrone, Paclitaxel ( rare risk), Vinblastine, Vincristine, Vindesine, Vinorelbine IRRITANTS Bleomycin, Carboplatin, Carmustine, Dacarbazine, Daunorubicin citrate liposomal, Doxorubicin liposomal, Etoposide, Ifosfamide, Oxaliplatin, Teniposide Non-antineoplastic agents that can cause irritation or extravasation include: Aminiophylline, Calcium, Dextrose 10%, Diazapem, Dobutamine, Dopamine, Doxycycline, Epinephrine, Erythromycin, Lorazepam, Nafcillin, Norephinephrine, Penicillin, Phenylephrine, Phenytoin, Piperacillin/Tazobactam, Potassium Solution, Promethazine, Contrast Media, Sodium Bicarbonate, TPN Solution, Vancomycin Signs and Symptoms of an Extravasation Versus vein irritation vs. Flare reaction Assessment Parameter Pain Redness Ulceration Swelling Blood Return Other Extravasation Vein Irritation Flare Reaction No pain. Immediate: severe pain or burning that last for minutes or hours and eventually subsides; usually occurs while the drug is being given and around the needle site Delayed: pain within 24 hours. Immediate blotchy redness around the needle site Delayed: may not occur at time of immediately Aching and tightness around vein The full length of the vein may be reddened or darkened. Immediate blotches or streaks along the vein, which usually subside within 30 minutes with or without treatment Develops insidiously; usually occurs 48-96 hours later. Severe swelling; usually occurs immediately or within 48 hours. Not usually. Not usually. Unlikely. Inability to obtain blood return; good blood return during drug administration. Change in the quality of the infusion and local tingling and sensory deficits. Usually. Unlikely, wheals may appear along the vein line. Usually. NA Urticaria PROCEDURE A. Extravasation 1. Stop infusion if infiltration/extravasation occurs 2. Notify physician, obtain antidote if indicated and ordered by physician. Note: a list of recommended antidotes/topical treatments can be found on the intranet under the pharmacy section. 3. Do not remove I.V. needle, immobilize extremity. 4. Disconnect tubing and gently aspirate any residual drug from needle/catheter with a small (1-3 cc) syringe. 5. House officer or primary physician will administer appropriate IV antidote into the extravasated area. If unable to administer antidote through the IV, the antidote should be given subcutaneously by the house officer or primary physician. 6. If appropriate, administer subcutaneous antidote in the extravasated area. a. Inject the antidote subcutaneously in multiple injections clockwise around/into the infiltrated area. b. Use 25-27 gauge needles and change the needle prior to each new injection. c. Repeat this process until the entire dose is injected in a full circle around the estimated borders of the extravasated area. 7. After injecting antidote or if no antidote applicable or ordered, remove IV needle, avoid applying direct pressure to site. 8. Apply topical ointment if ordered. 9. Apply ice pack for 15 minutes 4 times per day for the first 24-48 hours except for Vinca alkaloids (Vincristine, Vinblastine, Vinorelbine, Vindesine, Etoposide, Teniposide). For these 6 Vinca Alkaloids apply heat 4 times a day for the first 24-48 hours. 10. Assess the limb for pulses, capillary refill, and sensory and motor function. 11. Attempt to affected extremity elevated for 48 hours. 12. Measures borders of extravasated material and document width. 13. Document extravasation episode. Complete an incident report for infiltrations of chemotherapeutic drugs. Also chart the following: * * * * * * * date and time of event Needle/catheter size, type and location Drug sequence Nursing management Patient complaints, statements Appearance of site Physician notification and follow-up measures 14. Instruct the patient to report pain, redness, swelling, which continues more than 48 hours after event and/or at the first signs of the development of ulceration or necrosis at the site. Instruct patient to protect site from direct sunlight. B. Flare reaction (lack of pain swelling, and good blood return) 1. Flush the vein slowly with saline and watch for resolution of flare. 2. If no resolution get a physician’s order to administer hydrocortisone. For adults the dose is 25-50 mg IV followed by saline flush. 3. Once the flare reaction has resolved, slowly resume infusion of the drug. 4. For subsuquent infusions of same drug to same patient, premedication with antihistamines and or corticosteroids and slowing infusing rates may prevent further reactions. 5. Document episode including treatment and patient response. References: (2003) Paclitaxel package insert. Bristol Myers Squibb Company. (2005) Cancer Chemotherapy and Biotherapy Guidelines and Recommendations for Practice, Second Edition, Oncology Nursing Society. (2005) Martin, S., Cooper, T. and Sterling, J. Guide to Extravasation Management in Adult Patients. Hospital Pharmacy, Wolters Kluwer Health, Inc. (2005) Wilkes, G. and Barton-Burke, M. Oncology Nursing Drug Handbook. Jones and Bartlett.