National Extravasation Protocol
advertisement

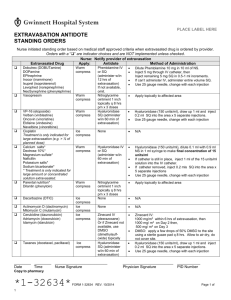
National Extravasation Protocol for Cancer Chemotherapy: A 10 step plan. Phase One – COOL & CALM 1. Stop the injection / infusion – Apply ColdA 2. Disconnect IV tubing – Leave cannula in situ - ‘Mark the Area’ with non soluble markerB. 3. V.L.T. assessmentC – Volume : Location : Time since occurrence. 4. Seek Expert Advice and plan management strategyD 5. Attempt AspirationE – Including if local facilities & expertise are available the ‘Flush Out’ technique. 6. Administer symptomatic treatment(s)F Phase Two – LOCALISE & NEUTRALISE 7. With intermittent coldG applied, attempt neutralisation if antidote exists and its application is appropriate to the individual clinical situationH. 8. Warm area to body temperature and continue antidotes if chronic application is applicableI. Phase Three – DILUTE & DISPERSE 9. Administer subcutaneous hyaluronidaseJ +/- fluidK, and warm the area to increase local blood supply and aid dispersionL. Phase Four – Report and Learn 10. Document the incident and action taken in the patient’s notes - Report the incident through both local and national schemes – Agree follow up and on going symptomatic and specific management as appropriate – Photograph the injury, acutely and at follow up. Notes: These notes are supported by 6 appendices: – Appendix 1. Definitions as used in the protocol Appendix 2. Flow chart version of the protocol Appendix 3. Details on the techniques mentioned in the protocol Appendix 4. Drug specific treatment information Appendix 5. Current classification table for chemotherapy agents Appendix 6. Example of the new, version 6 of the green card reporting forms for peripherally and centrally administered chemotherapy A. This is in the form of a flexible cold pack, such as those used for sports injuries, it should not be applied directly to the skin, i.e. wrap in a pillow case or tea towel. B. It is important to palpate the area and establish EITHER the size of the subcutaneous fluid pocket in a Type I extravasation; OR the boarders of the ‘soggy, spongy’ tissues in a Type II extravasation. These areas should be marked NOT the extent of the local reaction. C. V.L.T. Estimate the volume of the drug extravasated, Table 1 below may help in conjunction with measurements of the area measured in Note B, the practioner also needs to decide whether the volume is made up of a single drug or multiple drugs, and if so which drugs these are, and how much of the extravasated volume is made up of ‘carrier solution’. Location is important as certain areas are more able to accommodate larger volumes of fluid, e.g. the forearm, the more accommodating the compartment into which the extravasation has occurred, the more difficult it is to estimate the volume and the greater the risk of compartment syndrome developing. Time of the incident relative to the time of detection give an estimation of how much ‘natural’ diffusion will have taken place, i.e. the longer the time the greater the diffusion the more difficult it is to treat. Therefore all extravasation injuries should be treated as soon as detected, to minimise damage and maximise the chance of a successful outcome. Table 1: Approximate volume of material extravasated in relation to diameter of the injury and the level of intervention which may be appropriate. Volume Approximate Management Diameter* 0.1ml to 1.25ml Up to 17mm Watch and Wait 1.25ml to 2.5ml 17 to 30mm Topical low impact interventions Greater than Greater than Full blown no holes bared 2.5ml 30mm interventions * If diameter is measured within 15minutes of the acute extravasation so that minimal tissue diffusion will have occurred. Measurement should not include the inflamed surrounding tissue see Note B. above D. The trust or Network should appoint an extravasation coordinator; this individual should work with the local medicines information service to provide locally applicable advice. However if this individual is not available or if further advice is required; try the extravasation website or Andrew Stanley on 07976 960 474. E. Aspiration particularly for type II extravasation injuries is notoriously difficult, and produces limited success, if the local policy is to perform the wash out technique then it should be employed at this point in the pathway, patients should be returned to the protocol at stage three is dispersal of the residual wash out material is required or stage four to ensure the incident is correctly reported and followed up. Aspiration should be attempted using the ‘pin cushion’ technique, this technique is painful and distressing to the patient, therefore the marked area should be covered in Amitop or Emla cream and left for 30 minutes whilst topical anaesthesia is achieved. F. Symptomatic Management; this is about settling the local ‘cytokine’ cascade that has been triggered by the injury; the exact symptom management will depend on the extent and location of the injury and the nature of the patient. However practioners should considered topical and local treatments first, followed by central and oral interventions second, and intravenous and systemic treatments third and last, and only if required. Table 2 below details some of the topical, oral and intravenous interventions which have been used in extravasation injuries: Table 2: Topical Oral and Intravenous interventions to consider in the symptomatic management of an extravasation injury Agent Topical Hydrocortisone cream 1% Mode of Action Anti-inflammatory Crotamiton 10% cream(Eurax) Eurax-Hydrocortisone cream Heparinoid (Hirudoid) 0.3% cream Topical NSAID’s Ibuprofen or Diclofenac) (e.g. Oral and central Sodium Cromoglicate Ibuprofen (or alternative Anti-pruritic Combination of above Improve local circulation; used in the dispersal phase of an extravasation injury. Local pain relief Prevent degranulation of Mast cell and limit histamine release Systemic pain relief Dose & Schedule PRN in initial management then TDS TDS TDS QDS 500mg stat., may continue at 100mg QDS if clinically beneficial 400mg stat NSAID) Chlorpheniramine Paracetamol: can be escalated to Co-codamol 8/500’s and then to 30/500’s Morphine immediate release (Oramorph 10mg in 5ml or Sevredol 10mg tablets) Anti-histamine to ‘Mop up’ / ‘Dampen down’ released histamine. Adjuvant to the ibuprofen or for level 2 pain relief Level 3 pain relief Systemic – Intravenous or subcutaneously Diamorphine Sever, acute pain Hydrocortisone Chlorpheniramine Anti-inflammatory Alternative fast acting antihistamine. Alternative to 8mg po loading dose followed by 400mg tds 8mg stat followed by 4mg QDS 2 - QDS max 4g of paracetamol in 24hours. 10 or 20mg immediately followed by 10mg QDS prn 10mg IV, IM or s/c prn 200mg IV stat 10mg IV stat G. Intermittent cold is simply the use of the cold pack to restrict the local blood supply whilst the antidote, see Table 3, is instilled and for the following 24hours whilst it is allowed to work and or if repeated instillations of the antidote are required, this dose NOT apply to systemically administered intravenous antidotes, only those applied topically. H. The appropriate use of any antidotes should be dependant on the volume or believed volume of the extravasation, this is because the body processes natural homeostatic mechanisms for dealing with small volume toxic injuries. Table 1 above may help practioners in assessing the volume of material extravasated and in deciding the type of intervention which is appropriate. Table 3: Possible Topical, Subcutaneous Chemotherapy Extravasations. Antidote and Chemotherapy agents used with DMSO 50 to Epirubicin, Doxorubicin, 99%* Daunorubicin, Idarubicin, Mitoxantrone, Mitomycin C, [Topical] Dactinomycin Liposomal doxorubicin (Caelyx & Myocet), Liposomal Daunorubicin (DaunoXome), Amsacrine** Dexrazoxane Epirubicin, Doxorubicin, Daunorubicin, Idarubicin Systemic antidotes for Method of Use Apply 2 hourly to the effected area and allowed to dry without occlusion for the first 24hours then QDS for 7 to 14 days (21 days for liposomal preparations). Intravenously 1g/m2 on day 1 & 2 and 500mg/m2 on day 3 [Systemic] at approximately 24 hour intervals, and started within 6 hours of the extravasation. Mustine, Treosulfan, Busulfan Instil subcutaneously to the Cisplatin, Carboplatin, effected area by the ‘pin Oxaliplatin**, Arsenic** cushion’ technique. Sodium Thiosulphate (0.3%) [Subcutaneous] Desferrioxamine Cisplatin, Carboplatin, 500mg in 2ml of water for Oxaliplatin**, Arsenic** injection, instilled [Subcutaneous] subcutaneously to the effected area by the ‘pin cushion’ technique. Hyaluronidase Vincristine, Vinblastine, The IMMEDIATE use of 1500iu per Vindesine, Vinorelbine, hyaluronidase as an Paclitaxel, Docetaxel, nab “antidote” is recommended 2ml*** Paclitaxel (Abraxis)** essentially the practioners is [Subcutaneous] moving straight through to STEP 9 of the standard pathway. * The high the concentration available the greater and quicker it ‘solvent’ action is believed to work. ** Untried *** Diluted concentration after the addition of Water for Injection to the lyophilised powder. I. The chronic use of antidotes is only applicable to those applied topically and should be administered in sequence with topical symptomatic measures such as hydrocortisone cream. J. The subcutaneous hyaluronidase opens up the intracellular space around and throughout the affected area to allow for easier dispersal by natural body mechanisms. K. If the agent in question is hyper or hypo tonic to the surrounding tissues the addition of subcutaneous fluid (0.9% sodium chloride) will minimise the damage, furthermore if the pH of the extravasated fluid is close to the bodies natural buffer range 5.5 to 8.5 then dilution by a factor of 10 i.e. with 10ml if 1ml is believed to have extravasated or 100ml if the extravasation is as large as 10mls with s/c fluid will reduce the local pH by 1pH unit. The fluid should be administered by the technique known as hypodermoclysis. L. The area of the extravasation injury can be warmed using either a single use ‘thermal’ bandage, or a hot water bottle or microwavable ‘bean bag’ or using a small electric blanket. The chosen heat sores should not be applied directly to the extravasated area, but intermittently with a degree of gentle compression, the injury protected from the heat source via some plain gaze or the source wrapped in a pillow case or tea towel.