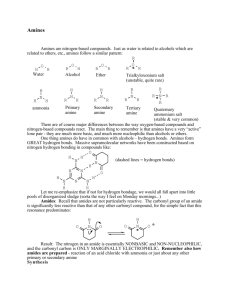
Chemical properties of amines:
advertisement

Organic chemistry and Biological chemistry for Health Sciences 59-191 Lecture 13 Chemical properties of amines: When amonia dissolves in water, the following equilibrium is established NH3 + H2O NH4+ + OH- A very similar equilibrium is established when an amine dissolves in water. RNH2 + H2O Kb = [R RNH3+ + OH[RNH3+][OH-] [RNH2] (Kb = base ionization constant) The higher the value of Kb is, the greater the dissociation of the base. Amines have Kb values slightly higher than amonia. Thus aliphatic amines are stronger bases than amonia. Like amonia, compounds with amino groups can also neutralize hydronium ions. This neutralization occurs very rapidly and essentially goes to completion at room temperature. Protonated amine cations can neutralize hydroxide ion and revert to amines. A combination of protonated amine and an anion make up an organic salt called an amine salt. Like the salts of amonium ions all amine salts of strong acids are more soluble in water than amine because the full charge carried by the ions of an amine salt can be much better hydrated by water molecules than the amine itself. The amino group is a solubility switch. By simply adding enough strong acids the solubility of amine can be switched on to give protonated amine (more soluble than amine). By quickly adding enough strong base you can bring the amine back out of solution. So adjusting the pH of the medium can change the solubility of complex compounds containing amino group almost instantly. Amides of carboxylic acids: Amides are neutral nitrogen compounds that can be hydrolyzed to carboxylic acids and amonia. Carbonyl nitrogen bond is called the amide bond. Amide bond is broken when an amide is hydrolyzed. Amides can be derived from amonia or amines. Simple amides are usually derived from amonia.