Amino acid metabolism
advertisement

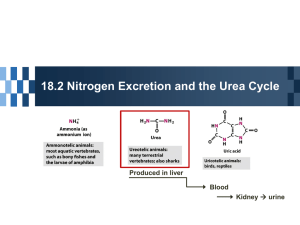
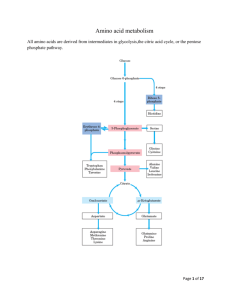
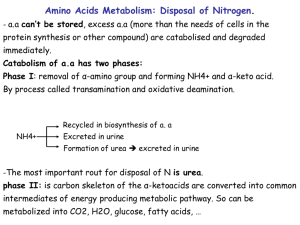
Amino acid metabolism Nitrogen balance Dietary protein amino acid pool protein synthesis N excretion (NH4+. urea) catabolism, biosynthesis normal N balance: N ingested = N excreted negative N balance: N ingested < N excreted positive N balance: N ingested > N excreted excess dietary amino acids (in excess over that required for protein synthesis) are not stored but are degraded and carbon skeletons used for glucose biosynthesis or energy production. proteins are constantly turning over and must therefore be constantly replaced by protein synthesis. This requires a steady supply of all 20 amino acids. Protein sparing: if carbohydrate or fat intake is inadequate, some dietary protein will be used for energy production, reducing availability of amino acids for protein synthesis. As carbohydrate and fats in diet increase, need for dietary protein decreases. Kwashiorkor: adequate caloric intake, but inadequate protein intake. Amino acid catabolism accounts for ~ 10% of energy requirement of adults When: excess protein in diet protein degradation exceeds demand for new protein starvation when carbohydrates are not available protein storing seeds such as beans, peas, etc. Glucogenic vs ketogenic amino acids ketogenic: yield AcCoA or AcAc as end products of catabolism glucogenic: are degraded to pyruvate or a member of the TCA cycle (succinylCoA, OAA, -ketoglutarate, fumarate). In absence of sugars, glucogenic amino acids permit continued oxidation of fatty acids by maintaining TCA cycle intermediates. glucogenic and ketogenic: yield both ketogenic and glucogenic products. ile, phe, tyr and trp are glucogenic. leu and lys are ketogenic. All others are glucogenic. N catabolism general strategy: removal of N from amino acid by transamination (generally first or second step of amino acid catabolic pathways) collection of N in glutamic acid deamination of glutamic acid with release of NH4+ Removal of NH4+ by : i. secretion; or ii. conversion to urea or other less toxic form. i and ii. Transamination; see text p 537 and fig 17.7. see also section 7.7, p212 on pyridoxal phosphate. iii. glutamate dehydrogenase (see p 533 for reaction) located in mitochondria operates near equilibrium iv. removal of NH4+ in liver by urea cycle and formation of urea in other tissues collection of N in glutamine or alanine for transport to liver formation of glutamine -glutamine synthetase (see fig 17.4) glutamine transported to liver or kidney where it is broken down to glutamate and NH4+ by glutaminase. in kidney NH4+ is secreted in urine with an anion such as -OH butyrate. + in liver NH4 is used to make urea. formation of alanine - "alanine glucose cycle" in skeletal muscle pyruvate acts as acceptor in transaminase reaction. Ala is transported to the liver where it undergoes transamination to yield pyruvate that is used for gluconeogenesis. The glucose is released and can return to muscle where is glycolytically degraded back to pyruvate. N metabolism in kidney glutamine converted to glutamate + NH4+ by glutaminase and NH4+ is secreted in urine along with an anion. during acidosis glutamine is shunted from liver to kidney to conserve bicarbonate in the liver (ie less NH4+ used for synthesis of urea) and extra NH4+ production in kidney is secreted with anions (eg ketone bodies) in urine. -ketoglutarate produced in kidney is used for production of HCO3-, which is released to blood (see p 563) and glucose. Urea cycle occurs in liver mito and cyto urea secreted in urine - up to 30 g/day source of N - glutamate dehydrogenase, glutaminase Reactions of: carbamylphosphate synthase - mito produces carbamyl phosphate from 2 ATP, CO2 and NH4+ committed step activated by N'Ac glutamate ornithine transcarbamylase - mito tightly coupled to carbamylphosphate synthase so that carbamyl phosphate is rapidly added to ornithine to form citrulline arginosuccinate synthtase - cyto aspartate transported into mito in exchange for glu. arginosuccinate lyase - cyto yields arginine and fumarate. fumarate used for synthesis of glucose (fumarate malate OAA PEP etc. arginase - cyto yields urea and ornithine ornithine transported back into mito absent from kidney which cannot make urea but is a source of arginine. Interorgan relationships in N metabolism Epithelial cells of intestine Several steps Glu’NH2 cittruline Glu’NH2 Liver Kidney cittruline Arg Arginine Arginine Several steps Urea Urea cycle Ornithine Several steps creatine glutamate To urine Muscle creatine P-creatine creatinine non-enzymatic Adapted from Devlin, Biochemistry with Clinical Corrleation 4th ed. Alanine - glucose cycle Muscle glucose 2 pyruvate -aa -ka 2 alanine glucose 2 alanine Liver glucose 2 alanine -ka 2 pyruvate -aa