Lecture 6
advertisement

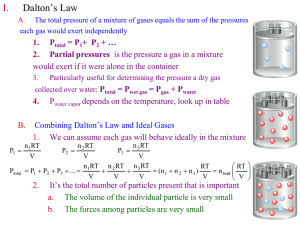
To Do… http://chem.illinois.edu Slides on the website (after each lecture) Lab tomorrow – review for the exam (due Friday, February 14 by 7 pm) Lon-Capa (HW3 Type 2 due Wednesday, February 12 by 7 pm). 1 Exam 1 Tuesday, February 18; 7:00 pm – 8:30 pm; 114 David Kinley Hall (DKH) Conflict: 2/18; 5:00 pm – 6:30 pm; 140 Burrill All free response. See me right away if you have a conflict with the exam and conflict times. 2 V vs. T(°C) (constant P, n) 3 V vs. T(K) (constant P, n) 4 Clicker Question Determine the size of a balloon filled with 4.00 moles of helium gas. Room conditions are 1.00 atm and 22C. a) 1.81 L b) 7.22 L c) 24.2 L d) 96.8 L 5 Clicker Question Determine the size of a balloon filled with 4.00 moles of helium gas. Room conditions are 1.00 atm and 22C. a) 1.81 L b) 7.22 L c) 24.2 L d) 96.8 L 6 Take a Molecular Level View 7 Kinetic Molecular Theory Gases consist of particles in constant random motion. Temperature is a measure of random kinetic energy. Pressure is due to collisions of gas particles with the container. 8 KMT (assumptions) Assume that gas particles exert no attractive forces. Assume the volume of the gas particles is negligible (zero). 9 PV/nT for Different Gases (all at 1 atm and 273K) O2: 0.08204 Latm/molK N2: 0.08206 Latm/molK NH3: 0.08083 Latm/molK Oxygen and nitrogen behave more ideally than ammonia. 10 V and n (KMT) 11 V and T (KMT) 12 P and V (KMT) 13 Egg in a Bottle Using liquid nitrogen to cool the bottle. What factors are changing? What factors are staying constant? Placing a flame in the bottle. What factors are changing? What factors are staying constant? 14