Practice Test #1
advertisement

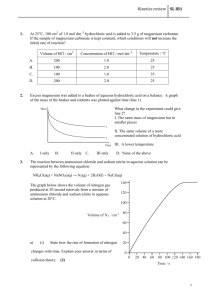
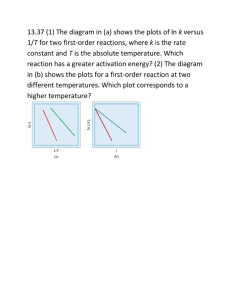
Practice Test#1 Chapter 1 - Kinetics Chapter 1 – Kinetics - Practice Test #1 Name - _________________ The answers to this test are posted online 1. 2. Consider the following reaction mechanism: step 1: M + X → step 2: MX + A → The chemical species MX is a(n) A. catalyst B. inhibitor X final product reaction intermediate 2.5 x 10-6 mol/s 5.0 x 10-6 mol/s C. D. Which of the following factors affect the rates of both homogeneous and heterogeneous reactions. A. B. nature of the reactants presence of a catalyst temperature of system concentration of reactants I and IV only II and III only C. D. II, III, and IV only I, II, III, and IV Which of the following equations represents an endothermic reaction? A. B. C. D. 5. C. D. 1.0 x 10-5 mol/s 1.3 x 10-6 mol/s I II III IV 4. + Consider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g) At a certain temperature the rate of decomposition of 2N2O5(g) is 2.5 x 10-6 mol/s. The rate of formation of NO2 is A. B. 3. MX D N2O4(g) 2H2(g) 2BrCl(g) 2H2(g) + 59 kJ → 2NO2(g) + O2(g) → 2H2O(l) + 572 kJ -29.3 kJ →Br2(g) + Cl2(g) + O2(g) → 2H2O(l) ΔH = -572 kJ Consider the potential energy diagram. The activation energy for the reverse reaction is A. B. 30 kJ 140 kJ PE (kJ) C. 170 kJ D. 200 kJ 170kJ 30kJ Progress of the reaction Practice Test#1 6. Consider the following mechanism: Step 1: Step 2: The reaction intermediate is A. B. 7. C. D. O3 ClO fastest and has the lowest activation energy. fastest and has the highest activation energy. slowest and has the lowest activation energy. slowest and has the highest activation energy. increasing the concentration of reactant(s). decreasing the concentration of the reactant(s). increasing the activation energy of the overall reaction. decreasing the activation energy of the overall reaction. Which of the following properties could be used to measure the rate of the following reaction in a open container. Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g) A. B. 10. Cl O2 + O2 + O2 A catalyst increases the rate of a reaction by A. B. C. D. 9. Cl + O3 → ClO O + ClO → Cl In a reaction mechanism, the rate determining step is the A. B. C. D. 8. Chapter 1 - Kinetics mass of Zn solubility of HCl Consider the following potential energy diagram: PE (kJ) Progress of the reaction The above diagram represents an A. B. C. D. exothermic reaction involving one step. exothermic reaction involving two steps. endothermic reaction involving one step. endothermic reaction involving two steps. C. D. concentration of Clcolour of the solution Practice Test#1 11. Which of the following are necessary for successful collisions to occur? I. II. III. A. B. 12. Chapter 1 - Kinetics Favourable geometry Sufficient energy Large ΔH I only I and II only Consider the following reaction: C. D. 2H2O2(aq) → II and III only I, II, and III 2H2O(l) + O2(g) When 1.0 g of KI is added to the H2O2, bubbles of O2 are produced at an increased rate, The KI is a A. B. 13. product catalyst C. D. reactant intermediate Consider the following I. Frequency of successful collision II. Volume of the reaction vessel III. Pressure of the system IV Mass of the system To increase the rate of a chemical reaction there must be an increase in A. B. 14. I only I and III only Consider the following reaction mechanism: Step1: ICl + H2 → HI + HCl slow Step 2: ICl + HI → HCl + I2 fast The species HCl is a A. B. C. D. product catalyst reactant reaction intermediate C. D. I, III and IV only I, II, III, and IV Practice Test#1 15. Chapter 1 - Kinetics Consider the following potential energy diagram: 125 PE (kJ) 100 75 50 25 Progress of the reaction The activation energy in the forward direction is A. B. 16. C. D. 75 kJ 125 kJ Consider the following reactions: I. N2 + O2(g) → 2NO(g) II. Mg(s) + O2(g) → 2MgO(s) III. CaCO3(s) + 2H+(aq) → Ca2+ (aq) + H2O(l) + CO2(g) Increasing the surface area will increase the reaction rate in A. B. 17. 25 kJ 50 kJ II only I and III only C. D. II and III only I, II, and III C. D. V3+ Fe3+ Consider the following reaction mechanism: Step 1: V3+ + Cu2+ → V4+ + Cu+ Step 2: Cu+ + Fe3+→ Cu2+ + Fe2+ slow The reaction intermediate is A. B. 18. Cu+ Cu2+ The rate of a chemical reaction can be expressed in A. B. C. D. grams per mole energy consumed per mole volume of gas per unit time mole formed per litre of solution Practice Test#1 19. Consider the following reaction: 2MnO4-(aq) + 5C2O42-(aq) 16H+(aq) → 2Mn2+(aq) + 10CO2(g) + 8H2O(l) The rate of decomposition of the oxalate ion is increased by A. B. 20. adding NaOH. removing CO2 C. D. adding a catalyst decreasing the pressure The minimum amount of energy needed to start a reaction is called the A. B. 21. Chapter 1 - Kinetics activation energy. energy of reaction. C. D. entropy of reaction reaction mechanism energy An 8.00g piece of magnesium was placed into 6.0 M HCl. After 25 s, 3.50 g of unreacted magnesium remained. The average rate at which magnesium was consumed is A. B. C. D. 0.14 g/s 0.18 g/s 0.32 g/s 4.50 g/s 22. In general rates double when the temperature is increased by 10 oC. The temperature of a reaction is increased by 40 oC. The rate will increase by a factor of A. 2 C. 8 B. 4 D. 16 23. Consider the following factors I. reactant particles collide II. sufficient kinetic energy is present III. a favourable geometry exists IV. catalysts are present Which combination of the above factors is required for all successful collisions? A. B. 24. C. D. I, II and III only I, II, III, and IV Consider the following reaction at constant temperature in an open system: MgCO3(s) + 2HCl(aq) → CO2(g) + H2O(l) + MgCl2(aq) Which of the following properties could be used to determine the reaction rate. A. B. 25. I only II and III only mass of the system pressure of the gas C. D. concentration of H2O concentration of MgCO3 Which combination of factors will affect the rate of the following reaction? MgCO3(s) + 2HCl(aq) → CO2(g) + H2O(l) + MgCl2(aq) A. temperature and surface area only B. temperature and concentration only C. concentration and surface area only D. temperature, concentration, and surface area only Practice Test#1 26. Chapter 1 - Kinetics As reactant molecules approach each other A. B. C. D. heat is released a reaction intermediate forms kinetic energy changes into potential energy potential energy changes into kinetic energy Consider the following potential energy diagram for the next three five questions. 2 PE 4 1 3 Progress of reaction 27. The interval representing ΔH for the reverse reaction is A. B. 28. 1 2 C. D. 3 4 1 2 C. D. 3 4 C. D. 3 4 The interval representing Ea for the forward reaction is A. B. 31. 3 4 The interval representing Ea for the reverse reaction is A. B. 30. C. D. The interval representing ΔH for the forward reaction is A. B. 29. 1 2 1 2 The interval representing the energy of the activated complex is A. B. 1 2 C. D. 3 4 Practice Test#1 32. When a catalyst is added to a reaction, ΔH will A. B. C. D. 33. Chapter 1 - Kinetics increase slowly remain constant decrease slowly increase rapidly due to the alternate pathway Consider the following reaction: Zn(s) + 2HCl(aq) → H2(g) + ZnCl2(aq) Data for the reaction is shown below: Time Mass of Zn (g) Volume of H2 (mL) Temperature (oC) 4.65 4.50 4.35 0 50 100 20 21 22 0 2 4 The rate of the reaction can be measured in units of A. B. 34. min/mL g/(mL)(oC) behaves as a catalyst supplies the activation energy is part of the rate determining step lowers the activation energy barrier Consider the following reaction: 2NO(g) + O2(g) → 2NO2(g) + 112 kJ ΔH for the above reaction is: A. B. C. D. 36. C. D. When a lit match is touched to the wick of a candle, the candle begins to burn. When the match is removed, the candle continues to burn, the match, A. B. C. D. 35. g/min g/mL positive and the reaction is exothermic negative and the reaction is exothermic positive and the reaction is endothermic negative and the reaction is endothermic Consider the following reaction: 2S(s) + 3O2(g) → 2SO2(g) + heat The rate of this reaction could be increased by A. B. C. D. decreasing the temperature adding a catalyst increasing the concentration of S decreasing the surface area of the S Practice Test#1 Chapter 1 - Kinetics 37. Consider the following reaction: ½H2 + ½I2 → HI ΔH = +28 kJ The activation energy for the formation of HI is 167 kJ. The activation energy for the decomposition of HI is A. 28 kJ C. 167 kJ B. 139 kJ D. 195 kJ 38. Some reactants are more reactive than others because of their activation energy Ea. What graph shows the relationship between Ea and rate. A. B. Ea Ea Rate C. D. Ea Rate 39. Ea Rate The activated complex is a chemical species that is A. B. 40. Rate stable and has low PE. stable and has high PE. As an activated complex changes into products, A. B. C. D. potential energy changes into kinetic energy. kinetic energy changes into potential energy. kinetic energy changes into activation energy. potential energy changes into activation energy. C. D. unstable and has low PE. unstable and has high PE. Practice Test#1 Chapter 1 - Kinetics Long Answer – Written response Questions PE Progress of the reaction 1. On the potential energy diagram above, clearly label the activation energy, heat of the reaction (∆H), and the energy of the activated complex. 2. Is the above reaction endothermic or exothermic in the forward direction? 3. On the graph below, draw the potential energy diagram for an exothermic reaction and label the activation energy. PE Progress of the reaction 4. a) Nitric oxide (NO) is involved in the decomposition of ozone by the following mechanism: Step 1: O3 + sunlight → O2 + O Step 2: O3 + NO → NO2 + O2 Step 3: NO2 + O → NO + O2 Write the net equation for the decomposition reaction b) Identify a catalyst c) Identify a reaction intermediate Practice Test#1 Chapter 1 - Kinetics d) What is the function of sunlight in this reaction? 5. Consider the following reaction: a) Explain why the reaction is likely to involve more than one step. b) A proposed mechanism for the above reaction is: Step 1: NO + H2 → N + H2O Step 2: ? Step 3: N2O + H2 → N2 + H2O 2NO + 2H2 → 2H2O + N2 Write the equation for step 2. 6. Define the term activation energy. 7. The combustion of coal, C, produces carbon dioxide and water according to the following equation: C(s) + O2(g) → CO2(g) + 394 kJ a) What is ∆H for this reaction? b) Using the collision theory, explain why a lump of coal does not react with oxygen at room temperature and pressure. c) Many coalmine disasters have resulted when a spark ignites coal dust in the air. Explain using the collision theory. 8. State two reasons why some collisions may not result in a chemical reaction. Practice Test#1 9. Chapter 1 - Kinetics A student wishes to monitor the rate of the following reaction: CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l) Identify two different properties that could be used to monitor the rate of the reaction. Describe and explain the changes that would occur. Property 1 Change and explanation Property 2 Change and explanation 10. An experiment is done to determine the rate of the following reaction: 2Al(s) + 6HCl(aq) → 3H2(g) + 2AlCl3(aq) The following data are collected Time (s) Mass of Flask + Contents (g) 0.0 30.0 60.0 270.230 270.200 270.170 Calculate the rate of consumption of Al in mol/min. 270.170 Practice Test#1 Chem 12 Chapter 1 - Kinetics Practice Test 1 Answers 1. Consider the following reaction mechanism: step 1: M + X → step 2: MX + A → The chemical species MX is a(n) A. catalyst B. inhibitor C. final product D. reaction intermediate MX D + X 2. Consider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g) At a certain temperature the rate of decomposition of 2N2O5(g) is 2.5 x 10-6 mol/s. The rate of formation of NO2 is A. B. C. D. 1.0 1.3 2.5 5.0 x x x x 10-5 mol/s 10-6 mol/s 10-6 mol/s 10-6 mol/s 3. Which of the following factors affect the rates of both homogeneous and heterogeneous reactions. I II III IV A. B. C. D. nature of the reactants presence of a catalyst temperature of system concentration of reactants I and IV only II and III only II, III, and IV only I, II, III, and IV 4. Which of the following equations represents an endothermic reaction? A. B. C. D. N2O4(g) 2H2(g) 2BrCl(g) 2H2(g) + 59 kJ → 2NO2(g) + O2(g) → 2H2O(l) + 572 kJ -29.3 kJ →Br2(g) + Cl2(g) + O2(g) → 2H2O(l) ΔH = -572 kJ Practice Test#1 Chapter 1 - Kinetics 5. Consider the potential energy diagram. The activation energy for the reverse reaction is A. B. 30 kJ 140 kJ PE (kJ) 30kJ 170kJ C. 170 kJ D. 200 kJ Progress of the reaction 6. Consider the following mechanism: Step 1: Step 2: Cl + O3 → ClO O + ClO → Cl + O2 + O2 The reaction intermediate is A. B. C. D. Cl O2 O3 ClO 7. In a reaction mechanism, the rate determining step is the A. B. C. D. fastest and has the lowest activation energy. fastest and has the highest activation energy. slowest and has the lowest activation energy. slowest and has the highest activation energy. 8. A catalyst increases the rate of a reaction by A. B. C. D. increasing the concentration of reactant(s). decreasing the concentration of the reactant(s). increasing the activation energy of the overall reaction. decreasing the activation energy of the overall reaction. 9. Which of the following properties could be used to measure the rate of the following reaction in a open container. Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g) A. B. C. mass of Zn solubility of HCl concentration of Cl- Practice Test#1 D. colour of the solution Chapter 1 - Kinetics Practice Test#1 Chapter 1 - Kinetics 10. Consider the following potential energy diagram: PE (kJ) Progress of the reaction The above diagram represents an A. B. C. D. exothermic reaction involving one step. exothermic reaction involving two steps. endothermic reaction involving one step. endothermic reaction involving two steps. 11. Which of the following are necessary for successful collisions to occur? I. II. III. A. B. C. D. Favourable geometry Sufficient energy Large ΔH I only I and II only II and III only I, II, and III 12. Consider the following reaction: 2H2O2(aq) → 2H2O(l) + O2(g) When 1.0 g of KI is added to the H2O2, bubbles of O2 are produced at an increased rate, The KI is a A. B. C. D. product catalyst reactant intermediate PE (kJ) Practice Test#1 Chapter 1 - Kinetics 13. Consider the following I. Frequency of successful collision II. Volume of the reaction vessel III. Pressure of the system IV Mass of the system To increase the rate of a chemical reaction there must be an increase in A. B. C. D. I only I and III only I, III and IV only I, II, III, and IV 14. Consider the following reaction mechanism: Step1: ICl + H2 → HI + HCl slow Step 2: ICl + HI → HCl + I2 fast The species HCl is a A. B. C. D. product catalyst reactant reaction intermediate 15.Consider the following potential energy diagram: 125 100 75 50 25 Progress of the reaction The activation energy in the forward direction is A. B. C. D. 25 kJ 50 kJ 100 kJ 125 kJ Practice Test#1 Chapter 1 - Kinetics 16. Consider the following reactions: I. N2 + O2(g) → 2NO(g) II. Mg(s) + O2(g) → 2MgO(s) III. CaCO3(s) + 2H+(aq) → Ca2+ (aq) + H2O(l) + CO2(g) Increasing the surface area will increase the reaction rate in A. B. C. D. II only I and III only II and III only I, II, and III 17. Consider the following reaction mechanism: Step 1: V3+ + Cu2+ → V4+ + Cu+ slow Step 2: Cu+ + Fe3+→ Cu2+ + Fe2+ slow The reaction intermediate is A. B. C. D. Cu+ Cu2+ V3+ Fe3+ 18. The rate of a chemical reaction can be expressed in A. B. C. D. grams per mole energy consumed per mole volume of gas per unit time mole formed per litre of solution 19. Consider the following reaction: 2MnO4-(aq) + 5C2O42-(aq) 16H+(aq) → 2Mn2+(aq) + 10CO2(g) + 8H2O(l) The rate of decomposition of the oxalate ion is increased by A. B. C. D. adding NaOH. removing CO2 adding a catalyst decreasing the pressure 20. The minimum amount of energy needed to start a reaction is called the A. B. C. D. activation energy. energy of reaction. entropy of reaction reaction mechanism energy Practice Test#1 Chapter 1 - Kinetics 21. An 8.00g piece of magnesium was placed into 6.0 M HCl. After 25 s, 3.50 g of unreacted magnesium remained. The average rate at which magnesium was consumed is A. B. C. D. 0.14 g/s 0.18 g/s 0.32 g/s 4.50 g/s 22. In general rates double when the temperature is increased by 10 oC. The temperature of a reaction is increased by 40 oC. The rate will increase by a factor of A. B. C. D. 2 4 8 16 23. Consider the following factors I. reactant particles collide II. sufficient kinetic energy is present III. a favourable geometry exists IV. catalysts are present Which combination of the above factors is required for all successful collisions? A. B. C. D. I only II and III only I, II and III only I, II, III, and IV 24. Consider the following reaction at constant temperature in an open system: MgCO3(s) + 2HCl(aq) → CO2(g) + H2O(l) + MgCl2(aq) Which of the following properties could be used to determine the reaction rate. A. B. C. D. mass of the system pressure of the gas concentration of H2O concentration of MgCO3 25. Which combination of factors will affect the rate of the following reaction? MgCO3(s) + 2HCl(aq) → CO2(g) + H2O(l) + MgCl2(aq) A. temperature and surface area only B. temperature and concentration only C. concentration and surface area only D. temperature, concentration, and surface area only Practice Test#1 Chapter 1 - Kinetics 26. As reactant molecules approach each other A. B. C. D. heat is released a reaction intermediate forms kinetic energy changes into potential energy potential energy changes into kinetic energy Consider the following potential energy diagram for the next three five questions. 2 PE 4 1 3 Progress of reaction 27. The interval representing ΔH for the reverse reaction is A. B. C. D. 1 2 3 4 28. The interval representing ΔH for the forward reaction is A. B. C. D. 1 2 3 4 29. The interval representing Ea for the reverse reaction is A. B. C. D. 1 2 3 4 Practice Test#1 Chapter 1 - Kinetics 30. The interval representing Ea for the forward reaction is A. B. C. D. 1 2 3 4 31. The interval representing the energy of the activated complex is A. B. C. D. 1 2 3 4 32. When a catalyst is added to a reaction, ΔH will A. B. C. D. increase slowly remain constant decrease slowly increase rapidly due to the alternate pathway 33. Consider the following reaction: Zn(s) + 2HCl(aq) → H2(g) + ZnCl2(aq) Data for the reaction is shown below: Time Mass of Zn (g) Volume of H2 (mL) Temperature (oC) 0 2 4 4.65 4.50 4.35 0 50 100 20 21 22 The rate of the reaction can be measured in units of A. B. C. D. g/min g/mL min/mL g/(mL)(oC) 34. When a lit match is touched to the wick of a candle, the candle begins to burn. When the match is removed, the candle continues to burn, the match, A. B. C. D. behaves as a catalyst supplies the activation energy is part of the rate determining step lowers the activation energy barrier 35. Consider the following reaction: 2NO(g) + O2(g) → 2NO2(g) + 112 kJ ΔH for the above reaction is: Practice Test#1 A. B. C. D. Chapter 1 - Kinetics positive and the reaction is exothermic negative and the reaction is exothermic positive and the reaction is endothermic negative and the reaction is endothermic 36. Consider the following reaction: 2S(s) + 3O2(g) → 2SO2(g) + heat The rate of this reaction could be increased by A. B. C. D. decreasing the temperature adding a catalyst increasing the concentration of S decreasing the surface area of the S 37. Consider the following reaction: ½H2 + ½I2 → HI ΔH = +28 kJ The activation energy for the formation of HI is 167 kJ. The activation energy for the decomposition of HI is A. B. C. D. 28 kJ 139 kJ 167 kJ 195 kJ 38. Some reactants are more reactive than others because of their activation energy Ea. What graph shows the relationship between Ea and rate. A. B. Ea Ea Rate C. Rate D. Ea Rate Ea Rate 39. The activated complex is a chemical species that is ` A. stable and has low PE. B. stable and has high PE. C. unstable and has low PE. D. unstable and has high PE. Practice Test#1 Chapter 1 - Kinetics 40. As an activated complex changes into products, A. B. C. D. potential energy changes into kinetic energy. kinetic energy changes into potential energy. kinetic energy changes into activation energy. potential energy changes into activation energy. Chemistry 12 PE 1999 Subjective Ea ∆H energy of activated complex Progress of the reaction 1. On the potential energy diagram above, clearly label the activation energy, heat of the reaction (∆H), and the energy of the activated complex. (3 marks) 2. Is the above reaction endothermic or exothermic in the forward direction? (1 mark) 3. On the graph below, draw the potential energy diagram for an exothermic reaction and label the activation energy. (2 marks) PE Ea 4. Nitric oxide (NO) is involved in the decomposition of ozone by the following mechanism: Step 1: O3 + sunlight → O2 + O Progress Step 2: O3 + NO → NO2 of + the O2 reaction Step 3: NO2 + O → NO + O2 a) Write the net equation for the decomposition reaction 2O3 + sunlight → 3O2 b) Identify a catalyst Practice Test#1 Chapter 1 - Kinetics NO c) Identify a reaction intermediate NO2 and O d) What is the function of sunlight in this reaction? (4 marks) Provides the activation energy 5. Consider the following reaction: 2NO + 2H2 → 2H2O + N2 a) Explain why the reaction is likely to involve more than one step. Reactions that have more than three reactant particles likely have mechanisms. b) A proposed mechanism for the above reaction is: Step 1: NO + H2 → N + H2O Step 2: ? Step 3: N2O + H2 → N2 + H2O Write the equation for step 2. NO + N → N2O 6. Define the term activation energy. The minimum energy required for a successful collision 7. The combustion of coal, C, produces carbon dioxide and water according to the following equation: C(s) + O2(g) → CO2(g) + 394 kJ a) What is ∆H for this reaction? (1 mark) ∆H + -394 kJ b) Using the collision theory, explain why a lump of coal does not react with oxygen at room temperature and pressure. (1 mark) The Ea is too high for the room temperature collisions to be successful. c) Many coalmine disasters have resulted when a spark ignites coal dust in the air. Explain using the collision theory. (2 marks) The spark provides the Ea, the reaction is exothermic and has a high surface area and an explosion results. 8. State two reasons why some collisions may not result in a chemical reaction. (2 marks) Practice Test#1 Chapter 1 - Kinetics Not sufficient energy Poor collision geometry 9. A student wishes to monitor the rate of the following reaction: CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l) Identify two different properties that could be used to monitor the rate of the reaction. Describe and explain the changes that would occur. Any two of the following. Property 1 Mass of CaCO3 Change and explanation Decreases as reactants are converted into products Property 2 Concentration of HCl Change and explanation Decreases as reactants are converted into products Property 3 Concentration of CaCl2 Change and explanation Increases as reactants are converted into products Property 4 Volume of CO2 gas Change and explanation Increases as reactants are converted into products Property 5 Mass of open system Change and explanation Decreases as gas escapes Property 6 Pressure of a closed system Change and explanation Increases as gas is produced 10. An experiment is done to determine the rate of the following reaction: 2Al(s) + 6HCl(aq) → 3H2(g) + 2AlCl3(aq) Time (s) Mass of Flask + Contents (g) 0.0 30.0 60.0 270.230 270.200 270.170 60.0 s 0.060 g H2 270.170 Practice Test#1 Chapter 1 - Kinetics Calculate the rate of consumption of Al in mol/min. 0.060 g H2 60.0 s x 60 s 1 min x 1mole H2 (3 marks) x 2.0 g 2 mol Al 3 mol H2 = 0.020 mol Al/min 1.00 g of Al is placed in a beaker and allowed to react for 12.00 minutes with 2.00 M HCl. If the rate of consumption of HCl is 0.250 g/min, calculate the amount of Al remaining. 12.00 min x 0.250 g HCl x 1 min 1.00 g - 0.740 g = 0.26 g 1 mol x 2 mol Al 36.5 g 6 mol HCl x 27.0 g 1 mole Note the loss of one sig fig. = 0.740 g