TimeTempAndRateOnConcen_Printable
advertisement

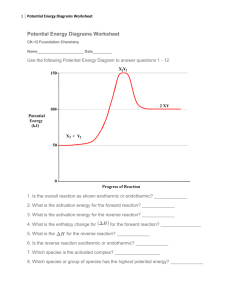
Chem 112 – Class Guide: The Effect of Time, Temperature and Rate on Concentration (Chapter 14, Sections 4-6) Chapter 14, Sections 4, 5 and 6 Learning Goals: Upon completion of Chapter 14, Sections 4-6, you should be able to determine the following: Identify the rate laws for first and second order reactions Calculate any variable in either the first or second order rate laws, given all other variables Identify the half-life equations for first and second order reactions Sketch an energy profile for endothermic or exothermic reactions Calculate activation energy using the Arrhenius Equation Identify the intermediates in a multi-step reaction Recognize that the slow step of a reaction limits overall reaction rate Chapter Reading Guide: Chapter 14, Sections 4 - 6 Section 4: THE CHANGE OF CONCENTRATION WITH TIME Read Chapter 14.4 Depending on the order of a reaction, there are different equations that are used in order to determine how much of a reactant is left after a specified time. For a first order reaction, the following pair of equations are used: 0.693 k [ A]t ln kt [ A]o t1 2 Where t1/2 is the half-life of the reactant, k is the decay constant, [A]o is the initial concentration of A, [A]t is the concentration of A after a certain time and t is the elapsed time. Please note the following two things: 1.) [A]o is ALWAYS greater than [A]t; 2.) t is rarely ever equal to t1/2. For a second order reaction, the following pair of equations are used: t1 2 1 k[ A]o 1 1 kt [ A]t [ A]o Where t1/2 is the half-life of the reactant, k is the decay constant, [A]o is the initial concentration of A, [A]t is the concentration of A after a certain time and t is the elapsed time. Please note the following two things: 1.) [A]o is ALWAYS greater than [A]t; 2.) t is rarely ever equal to t1/2. Try Practice Exercise 14.7 Try Practice Exercise 14.8 Try Practice Exercise 14.9 Section 5: TEMPERATURE AND RATE Read Chapter 14.5 The activation energy of a reaction is the amount of energy it takes to start a reaction. Activation energies are best visualized by looking at energy profiles that often look like the following: Activation energy is often abbreviated Ea and the change in energy is often called ΔE. Remember that a positive ΔE is an endothermic reaction and a negative ΔE is an exothermic reaction. In the example energy profile, the reaction would be an exothermic reaction, since the energy of the products is lower than that of the reactants. There are also ways to calculate the activation energy of a reaction. The first is to use the Arrhenius equation: Ea k Ae RT Where k is the rate constant of the reaction, A is called the frequency factor, Ea is the activation energy, T is the temperature and R is the universal gas constant (8.314 J/mol·K). One can also expand the Arrhenius equation to look like: k E 1 1 ln 1 a k2 R T2 T1 Where k1 is given at some temperature T1 (in Kelvin) and k2 is given at some temperature T2. Try Practice Exercise 14.10 Try Practice Exercise 14.11 Section 6: REACTION MECHANISMS Read Chapter 14.6 Reactions do not often occur in one step; they take several different steps to go from initial reactants to final products. The steps by which a multi-step reaction occurs is called a reaction mechanism. If a reaction DOES take place in one step, it is called an elementary reaction and can further identified by how many reactants there are in an elementary reaction. If there is one reactant, the reaction is called a unimolecular reaction. If there are two, it is called bimolecular and if there are three, it is called a termolecular reaction. In a multistep reaction, there are often more molecules involved in the process than what the net of the mechanism is. Any molecule that is a product of one step in a mechanism and is then a reactant of a subsequent step is called an intermediate. Intermediates are never present in the overall net reaction, but are formed during the course of the reaction. For a multi-step reaction, the rate law is always based off of the slow step of the reaction. Try Practice exercise 14.12 Try Practice exercise 14.14 Learning Resources Chapter Learning Goals Chapter 14, Sections 4 - 6 Learning Goals Pre Class Assignment: This assignment must be completed prior to the next class. Check your syllabus for the exact due date and time. Complete the pre class assignment (http://berks.psu.edu/clt/chem112/TimeTempAndRateOnConcen_HW.docx) Submit a copy to the dropbox located in ANGEL called “Pre Class Assignment Submission: The Effect of Time, Temperature and Rate on Concentration” End of Chapter Problems: Practice with these problems if you are having difficulty with any of the concepts covered in this class guide AFTER we have met in class. If you cannot easily complete these problems, seek help from your instructor, your mentor or the learning center Chapter 14: 43, 45, 57, 63