Spring 2008 Exam 2 answers
advertisement

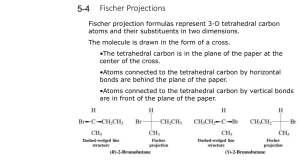
Chemistry 320 Spring 2008 Exam 2 1. Draw Fischer projections for the four D-aldopentoses. Circle the ones which would produce optically active aldaric acids upon oxidations with warm nitric acid. [note: there is no need to provide the names of these compounds simply draw the four stereoisomers]. (10 pts) 2. In chapter 18 you learned about using acetals as a protecting group for aldehydes and ketones. Below is a synthesis that will only work with such a protecting group. What set of reactions would you use to complete this transformation? (5 pts) First you protect the ketone as a diethyl acetal. The Grignard reagent then has only one place to react, the ester. After you are sure the Grignard has finished reacting, you need to neutralize the reaction. Luckily these conditions also remove the acetal protecting group and free your ketone. – you may want to allow the aqueous acid a longer time to react, and you may need to heat the mixture to insure that all of the acetal protecting group is removed. 3. Use orbital diagrams to explain why E,E-2,4-hexadiene undergoes a photochemical cyclization to produce only cis-3,4-dimethylcyclobutene . (5 pts) In a photochemical reaction, an electron is excited from the HOMO to the LUMO. This orbital now becomes the orbital which controls the reaction stereochemistry. Since both positive lobes of the end orbitals are pointing up, they would rotate in through a disrotary ring closure to produce the cis-product shown above. 4. Show the product(s) in five of the following reactions. Make sure you clearly indicate stereochemistry where appropriate. (50 pts) 5. Show all of the steps of the mechanism for one of the following reactions. Make sure you indicate which steps are reversible and which are irreversible (arrows will work). (15 pts) 6. Show a synthesis for one of these compounds. You may start with any alcohols containing four or less carbons, bromobenzene, any inorganic reagents, and any solvents you need. (10 pts) Multiple Choice: (3 pts each) Choose the one best answer. 7. Which of these aldopentoses are a pair of enantiomers? a. b. c. d. e. I and II I and III II and III All of these are enantiomers of each other None of these are enantiomers of each other 8. Which organic product is formed by this reaction? 9. How many stereoisomers exist for the following molecule? OH OH OH a. 2 b. 3 c. 4 d. 6 e. 8 10. A Diels-Alder reaction is an example of a a. concerted pericyclic reaction b. an electrocyclic reaction c. a cycloaddition reaction d. A and B e. A and C 11. The compound D-xylose (an aldopentose) is classified as a D sugar because a. it rotates plane-polarized light in a clockwise direction. b. it rotates plane-polarized light in a counterclockwise direction. c. the hydroxy group on carbon 2 is to the right of the carbon chain in its Fischer projection. d. the hydroxy group on carbon 4 is to the right of the carbon chain in its Fischer projection. e. the hydroxy group on carbon 5 is to the right of the carbon chain in its Fischer projection. 12. Which is an intermediate in the formation of an ester from ethanoyl chloride and ethanol? d. A. and C. e. B. and C. 13. Which is false regarding a reaction mechanism? a. It is possible to prove that a reaction follows a particular mechanism. b. A reaction mechanism helps visualize a stepwise process of electron flow and bond cleavage and formation for a reaction. c. Experimental evidence may rule out a mechanistic pathway. d. Electron movement (flow) occurs from a nucleophile to an electrophile. e. Total charge is always conserved in any given step of a reaction mechanism. 14. Which of these acid derivatives is a good starting material for the reaction below? O ? Methanol R O a. anhydride b. acid chloride c. amide d. a and b e. b and c 15. What is the product of this reaction? O NaBH 4 , CH 3 OH H O O OH OH H H a. b. O OCH 3 d. c. O HO HO H e. 16. Show the reactants and reagents that would effect a synthesis of the following compound. OH H3 O + O O H a. 1) Mg, ether 2) CH 3CHO 3) H3 O + b. 1) MgBr CH 3CH 2 Br 1) CH 3CH 2 Br 2) MgBr 3) H3 O + OH H 3) H3 O + c. d. 1) Mg, ether CH 3CH 2 Br O 2) H 3) H3 O + e. O 2)