File - Wk 1-2
advertisement

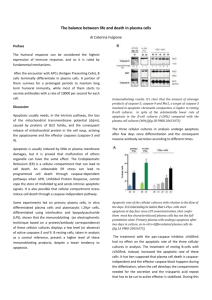
Pathology of apoptosis 1. Outline the sequence of morphological changes that occur in cells undergoing apoptosis Apoptosis is a pathway of cell death that is induced by a tightly regulated intracellular program in which cells destined to die activate enzymes that degrade the cells own nuclear DNA, as well as nuclear and cytoplasmic proteins. The cells plasma membrane remains intact, but its structure is altered in such a way that the apoptotic cell becomes an avid target for phagocytosis. Phagocytosis is required so dead cell can be rapidly cleared before its contents can be leaked out and damage surrounding cells. Morphological changes to a cell undergoing apoptosis include; - Cell Shrinkage – cell smaller in size, dense cytoplasm, organelles tightly packed together - Nuclear condensation – “most characteristic feature of apoptosis” – chromatin aggregates peripherally under the nuclear membrane into dense masses - Nuclear Fragmentation – nucleus itself may break up, producing two or more fragments - Formation of cytoplasmic blebs and apoptotic bodies – apoptotic cell first shows surface blebbing, then undergoes fragmentation into membrane bound apoptotic bodies composed of cytoplasm and organelles, with or without nuclear fragments - Phagocytosis of apoptotic cells or cell bodies, usually by macrophages – Expression of phosphatidylserine on the outer plasma membrane of apoptotic cells/ apoptotic bodies may express thrombospondin, an adhesive glycoprotein/ proteins secreted by phagocytes act to opsonize the cells for phagocytosis. This results in removal of apoptotic cells, with adjacent healthy cells migrating or proliferating to replace the space occupied by the now deleted apoptotic cell. NB. Plasma membrane remains intact during apoptosis, where as plasma membrane damage occurs during necrosis. This results in cell swelling rather than shrinkage 2. Identify typical apoptotic cells and bodies histologically and in electron micrographs The cell demonstrates classical features of apoptosis, with blebbing of the nucleus and cytoplasm in preparation for phagocytosis of the apoptotic debris by phagocytes and other surrounding cells. 3. List the circumstances in which cell death by apoptosis might be expected Apoptosis occurs normally in many situations, and serves to eliminate unwanted or potentially harmful cells, or cells no longer required. Important physiological situations where death by apoptosis may occur include - Programmed destruction of cells during embryogenesis - Hormone dependent involution in adults ie endometrial cell breakdown in menstruation - Cell deletion in proliferating cell populations ie rapidly dividing cells of GIT epithelia - Death of cells that have served there purpose ie neutrophils and lymphocytes - Elimination of potentially harmful self-reactive lymphocytes ie maturation - Cell death as a defence mechanism against virus infection mediated by CD8 cells Pathologic states also are responsible for cell death - Injurious stimuli ie radiation and cytotoxic chemicals - Heat, hypoxia, stress - Viral diseases - Loss of growth factors or hormones 4. Outline the biochemistry and molecular biology of apoptosis with particular reference to cell shrinkage and condensation, cell budding, DNA fragmentation, receptor-mediated phagocytosis, tissue transglutaminase activity, and the roles of c-myc, p53, bcl-2 and caspases Apoptotic cells exhibit a distinct array of biochemical changes that underlie the structural changes described earlier. Some specific features include; Protein cleavage A feature of apoptosis is protein hydrolysis involving the activation of several cysteine proteases called caspases ( ‘c’ from cysteine protease; ‘aspase’ from the ability of the enzyme to cleave after aspartic acid residues). There are approximately 10 members of the caspase family and they can be divided into two functional groups; 1. Initiator caspases – cleave inactive pro-forms of effector caspases, thus activating them. Include caspase-2, -8, -9, -10 2. Effector (executioner) caspases – cleave other protein substrates within the cell to trigger apoptotic processes. Include caspase-3, -6, -7 Caspases are regulated at the post-translational level, ensuring that they can be rapidly activated. They exist initially in an inactive form (pro-caspase or zymogen) and must undergo an activating cleavage for apoptosis to be initiated. The important thing is that caspases can be activated by other caspases as well as themselves. Once initiator caspase is cleaved and activated, the death program is set in motion via the rapid and sequential activation of other (executioner) caspases. Caspases then activate other cellular proteins, such as Lamins – breakdown nuclear scaffold and cytoskeleton DNAses – degrade These changes underlie the nuclear and cytoplasmic structural alterations seen in apoptotic cells DNA breakdown Characteristic breakdown of DNA into large 50-300kb pieces, and then internucleosomal cleavage of DNA by endonuclease into oligonucleosomes of 180200bp. Fragments may be visualised on agarose-gel electrophoresis. Endonuclease activity the basis for detection of apoptosis Phagocytic Recognition Apoptotic cells express phosphatidylserine in the outer layers of their plasma membranes, having flipped out from the inner layers. Also, apoptotic bodies may express thrombospondin, an adhesive glycoprotein. Other proteins secreted by phagocytes may also act to opsonize the cells for phagocytosis. These alterations permit the early recognition of dead cells by macrophages, for quick removal without further damage and with out the release of pro-inflammatory mediators MECHANISMS OF APOPTOSIS Apoptosis is induced by a cascade of molecular events that can be initiated in a number of distinct ways. The end result of this initiation is the culmination of activated caspases. The process of apoptosis may be divided into an Initiation Phase, in which caspases become catalytically active, and an Execution phase, during which these enzymes act to cause a series of events leading to cell death. Initiation occurs primarily via signals from two distinct pathways; 1. The Extrinsic (Death Receptor-Initiated) Pathway. This pathway is initiated via the engagement of cell surface receptors on a variety of cells. These include the Tumour Necrosis Factor (TNF) and Fas family of receptors. These receptors contain a protein cytoplasmic domain. Upon engagement by ligand ie FasL or TNF, receptor molecules aggregate resulting in the formation of a protein rich cytoplasmic domain known as a Death Domain. This provides a binding site for an adapter protein that also contains a death domain. For Fas receptors, this is known as FADD (Fas-Associated Death Domain). For TNF receptors, they associate with TRADD (TNF Receptor-associated Death Domain), which in turn binds FADD. FADD attached to death receptors in turn binds inactive pro-caspase-8. Multiple procaspase-8 brought into close proximity with one another cleave one another to produce active caspase-8 (initiator caspase) which then goes on to activate other caspases that activate the enzymes that mediate the execution phase of apoptosis. 2. The Intrinsic (Mitochondrial) Pathway This pathway is related to an increased mitochondrial membrane permeability resulting in the release of pro-apoptotic molecules into the cytoplasm. One way this happens is by the withdrawal of growth factors or hormones. In the mitochondria, growth factors and other survival signals stimulate the production of anti-apoptotic molecules such as the members of the Bcl family. The Bcl family are a large family of proteins, some with anti-apoptotic properties and others with pro-apoptotic properties. The two main anti-apoptotic ones are Bcl-2 and Bcl-X. They reside in the mitochondrial membrane and are believed to govern the inactivation of mitochondrial transition pores. When cell is deprived of survival signals or placed under stress, production of these proteins is lost, and they are replace by pro-apoptotic members such as Bax, Bak, Bid, Bik etc. When Bcl-2/-X levels decrease and levels of the others rise, mitochondrial permeability increases and proteins that activate the caspase cascade leak out. This includes Cytochrome c (cyt-c). Cyt-c binds to a protein called Apaf-1(Apoptosis Inducing Factor-1) and this complex activates pro-caspase-9, an executioner caspase. Bcl-2/-x directly inhibits Apaf-1 activation. Other mitochondrial proteins that are pro-apoptotic, such as Apoptosis Inducing Factor (AIF), enter the cytoplasm where they bind to and neutralize Inhibitops or Apoptosis (IAPs). The normal function of IAPs is to block caspase activation Other methods of inducing the apoptosis signal to activate caspases, DNA Damage Mediated Apoptosis Exposure of cells to radiation or chemotherapeutic agents induces apoptosis that involves the tumour suppressor gene p53. p53 accumulates when DNA is damaged and arrests the cell cycle (at the G1 phase) so repair can take place. If repair process fails, p53 can trigger apoptosis. It does this through the production of pro-apoptotic Bcl family proteins (Bax, Bak) and these activate caspases that cause apoptosis. If p53 is mutated or absent in certain disease states it becomes incapable of inducing apoptosis favouring cell survival. Cytotoxic T-Lympthocyte (CD8) Mediated Apoptosis. CD8 cells recognise foreign antigens on the surface of infected cells. Perforin is a cytolytic protein found in the granules of T-cells and NK cells. Upon degranulation, perforin inserts itself into the target cell's plasma membrane, forming a pore (Polyperforin channel). Through this pore, a second group of proteins and enzymes (Granyzymes - B) enter the cytosol and activate caspases (namely caspase-3, -7). CD8 cells also posses the FasL on their surface, capable of engaging Fas receptors and inducting Death-Receptor mediated apoptosis The final phase of Apoptosis, the Execution phase is mediated by a proteolytic cascade courtesy of activated caspases. Once initiator caspases have been cleaved and activated from their inactive pro-caspase form, they then go on to activate executioner caspases. Executioner caspases (which can also be activated directly ie Granzyme B and p53) then go on to act on many cellular components. They directly cleave cytoskeletal and nuclear matrix components and activate latent cytoplasmic endonucleases and DNAses. This results in intracellular degradation, including fragmentation of nuclear chromatin and breakdown of the cytoskeleton. The end result is the formation of apoptotic bodies containing intracellular organelles and other components. Apoptotic bodies also express new ligands which can be used for recognition and binding for uptake by phagocytic cells. During early stages of apoptosis, dying cells secrete chemokine that act to attract phagocytes to the area. Also, apoptotic cells have marker molecules on their surface which facilitates early recognition and uptake by phagocytes (ie phosphatidylserine). Phagocytes also secrete substances that bind specifically to apoptotic cells to opsonise them for phagocytosis. Prompt clearance of apoptotic cells prevents them from undergoing secondary necrosis and releasing their cellular contents, having an inflammatory effect.