Chapter 21
advertisement
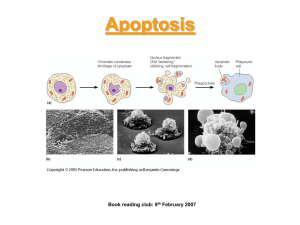
Chap. 21 Stem Cells, Cell Asymmetry, and Cell Death Topics • Cell Death and Its Regulation Goals • Learn the basic mechanism of apoptosis and its regulation. • Learn the basic roles of neurotrophins and apoptosis in wiring of the nervous system. • Learn the functions of caspases in apoptosis. Overview of Apoptosis Apoptosis (programmed cell death) is a cell fate that is essential in some developmental programs. Apoptosis is highly regulated. It can be induced by withdrawal of trophic factors, which signal cells to stay alive. Alternatively, signals (e.g., death signals like tumor necrosis factor) trigger apoptosis. The structural changes that occur during apoptosis are morphologically distinct from changes that occur due to cell death via necrosis (Fig. 21.30). In necrosis cells typically burst and release their contents outside. This damages surrounding cells and can lead to inflammation. In apoptosis, cells shrink, condense, and fragment without the release of cell contents. Cell fragments known as apoptotic bodies are later phagocytosed. Three types of proteins control cell death. Killer proteins initiate apoptosis, destruction proteins digest DNA and other cellular components, and engulfment proteins are required in phagocytosis. Overview of Apoptosis Pathways Apoptosis pathways discovered by genetic studies in worms and mammals are shown in Fig. 21.33. Proteins such as the CED-9 and Bcl-2 homologs serve as sensors of signals that regulate apoptosis. In the presence of a trophic factor, these regulators block the activation of adaptor proteins (CED-4 and Apaf-1) and thereby prevent effector proteins (the CED-3, Casp9, and Casp3 proteases) from becoming activated. These effectors are enzymes known as caspases. When activated, caspases cause cells to undergo apoptosis by cleaving key intracellular substrates resulting in a cascade of events leading to disassembly of cellular structures and death. Apoptosis in Motor Neuron Development In the development of the nervous system, motor neurons and other neurons must establish connections between themselves and tissues they innervate, such as muscle. Typically, more neurons grow initially and migrate towards the target tissue than ultimately will survive. Those that fail to form connections to the target tissue are selected for apoptosis. Grafting studies performed with embryos showed that the formation of connections and survival of developing neurons depends on the quantity of neurotrophic factors they receive from the target field tissue they will innervate (Fig. 21.35). The greater the quantity of factors received, the higher the percentage of cell survival. Neurotrophins and Their Receptors Neurotrophic factors (neurotrophins) include nerve growth factor (NGF), neurotrophin-3 (NT-3), and brain-derived neurotrophic factor (BDNF). The receptors for neurotrophins are RTKs named Trks. Trk receptors reside on the cell surface of neurons and bind to their ligands released from the tissues that they will eventually innervate. Studies with knockout mice have established some of the roles of neurotrophins in nervous system development (Fig. 21.36). When the genes encoding NGF or its receptor TrkA are knocked out, mice selectively fail to form nociceptive (pain-sensing) neurons that innervate the skin. Knockout of the genes encoding NT-3 or its receptor TrkC inhibits the formation of propioceptive neurons that innervate skeletal muscle fibers. These studies show that neurotrophin signaling is required for developing neurons to survive instead of undergoing apoptosis. Caspase Activation in the Absence of Trophic Factors The mechanism by which caspases are activated in the absence of a trophic factor (e.g., NGF) is illustrated in Fig. 21.38. In the absence of the trophic factor, the proapoptotic protein Bad inhibits the anti-apoptotic protein Bcl-2. As a result, Bcl-2 cannot inhibit the activity of the Bax pro-apoptotic protein. This results in release of cytochrome c (Cyt c) from mitochondria. Cyt c binds to the Apaf-1 adaptor protein, and this triggers procaspase 9 to undergo activation. Caspase 9 then cleaves and activates procaspase 3. Caspase 3 cleaves substrates leading to alterations that result in cell death. Caspase activation and cell death can also be triggered by binding of death signals, such as tumor necrosis factor (TNF) to cells (not shown). Inhibition of Caspase Activation by Trophic Factors In the presence of a trophic factor, caspases are maintained in their inactive pro-forms (Fig. 21.38). In neurons, NGF causes Bad to be phosphorylated and inactivated via the PI-3 kinase/PKB signaling pathway. Under these conditions Bcl-2 can inhibit the activity of Bax. This prevents Cyt c release from mitochondria and blocks the activation of procaspase 9 by Apaf-1.