U3.1 Ionic Compounds
advertisement
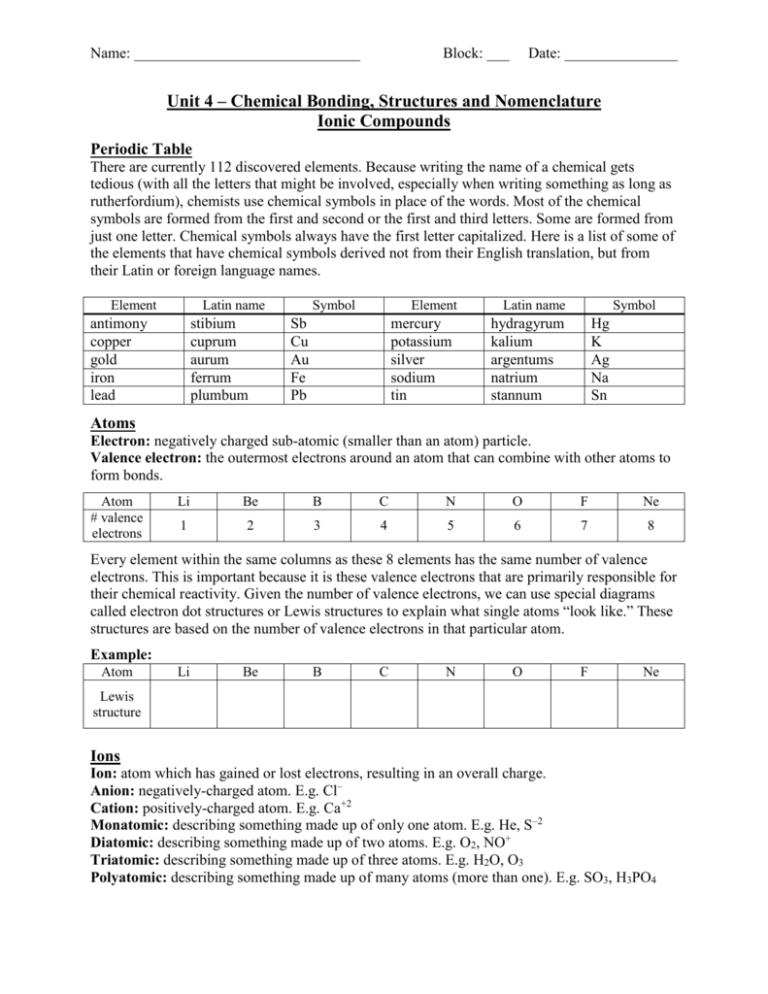
Name: ______________________________ Block: ___ Date: _______________ Unit 4 – Chemical Bonding, Structures and Nomenclature Ionic Compounds Periodic Table There are currently 112 discovered elements. Because writing the name of a chemical gets tedious (with all the letters that might be involved, especially when writing something as long as rutherfordium), chemists use chemical symbols in place of the words. Most of the chemical symbols are formed from the first and second or the first and third letters. Some are formed from just one letter. Chemical symbols always have the first letter capitalized. Here is a list of some of the elements that have chemical symbols derived not from their English translation, but from their Latin or foreign language names. Element Latin name antimony copper gold iron lead stibium cuprum aurum ferrum plumbum Symbol Element Sb Cu Au Fe Pb mercury potassium silver sodium tin Latin name Symbol hydragyrum kalium argentums natrium stannum Hg K Ag Na Sn Atoms Electron: negatively charged sub-atomic (smaller than an atom) particle. Valence electron: the outermost electrons around an atom that can combine with other atoms to form bonds. Atom # valence electrons Li Be B C N O F Ne 1 2 3 4 5 6 7 8 Every element within the same columns as these 8 elements has the same number of valence electrons. This is important because it is these valence electrons that are primarily responsible for their chemical reactivity. Given the number of valence electrons, we can use special diagrams called electron dot structures or Lewis structures to explain what single atoms “look like.” These structures are based on the number of valence electrons in that particular atom. Example: Atom Li Be B C N O F Ne Lewis structure Ions Ion: atom which has gained or lost electrons, resulting in an overall charge. Anion: negatively-charged atom. E.g. Cl– Cation: positively-charged atom. E.g. Ca+2 Monatomic: describing something made up of only one atom. E.g. He, S–2 Diatomic: describing something made up of two atoms. E.g. O2, NO+ Triatomic: describing something made up of three atoms. E.g. H2O, O3 Polyatomic: describing something made up of many atoms (more than one). E.g. SO3, H3PO4 Typically, when elements with a low number of valence electrons become ions, they prefer to lose those electrons and become cations. These atoms are called metals. When atoms with a high number of valence electrons become ions, they prefer to gain electrons and become anions. These elements are known as non-metals. When possible, these atoms that would form anions want to obtain a total of 8 valence electrons. Naming Monatomic Metal Ions To name monatomic metal ions, use the name of the metal and add the word “ion.” For elements in Group 1 (left-most column of periodic table), they all form +1 ionic charges. For elements in Group 2 (second-from-left column), they all form +2 ionic charges. E.g. Sodium metal forms the sodium ion (Na+). Aluminum metal forms the aluminum ion (Al+3). Stock System of naming metal ions: If the metal ion has more than one possible charge, the charge is indicated by a Roman numeral, in parentheses, immediately following the name. E.g. Mn+2 = manganese(II) ion. V+3 = vanadium(III) ion. Practice: Write the names of the following ions using the Stock system of notation. a) Pt+2 b) Au+ c) Cr+6 d) Hg 22 Naming Monatomic Non-Metal Ions To name monatomic non-metal ions, take the original ending of the element’s name and put on an “-ide” ending. (The ending “-ide” means that the ion has a negative charge.) Element Name fluorine chlorine bromine iodine oxygen sulfur nitrogen phosphorus Element Symbol F Cl Br I O S N P Ion Name fluoride chloride bromide iodide oxide sulfide nitride phosphide Ion Symbol F– Cl– Br– I– O–2 S–2 N–3 P–3 The most common ionic charge for elements in Group 15 (the nitrogen group) is –3. The most common ionic charge for elements in Group 16 (the oxygen group) is –2. The most common ionic charge for elements in Group 17 (starting with fluorine) is –1. Constructing the Formula of an Ionic Compound (given the name of the compound) Ionic compound: compound made of ions. (In Chem 11, they are typically metal/non-metal compounds.) Compounds are neutral molecules. Therefore the sum of the “+” ion charges in the molecule must equal the sum of the “–” ion charges in the molecule. Translating a chemical name into a chemical formula can be done following three rules: Name: ______________________________ Block: ___ Date: _______________ 1. Write the formula for the cation first and write the formula for the anion second. (In the name, the cation is always written first and the anion is always second.) a. Lead(IV) oxide is translated as Pb+4 O–2 2. “Criss-cross” the numbers in front of the charges on the ions. If no number is shown, use “1.” The reason for criss-crossing is to make the total “+” charges = total “–” charges. a. Pb24 O 4 2 3. Tidy up the formula. If both subscripts can be evenly divided by “2”or “3,” do so. Omit the superscripted charges. Omit any subscript which is a “1.” a. Pb24 O 4 2 Pb14 O 2 2 PbO2 Example: barium chloride: barium ion = Ba+2; chloride ion = Cl– Criss-cross the charges – Ba 12 Cl 2 – eliminate charges and subscripts of “1”: BaCl2 iron(III) oxide: iron(III) ion = Fe+3; oxide ion = O–2 Criss-cross the charges – Fe 2 3 O 32 – eliminate charges: Fe2O3 mercury(I) bromide: mercury(I) ion = Hg 22 ; bromide ion = Br– Criss-cross the charges – Hg 22 1 Br21 – eliminate charges and subscripts of “1”: Hg2Br2 (Note: mercury(I) ion = Hg 22 ; mercury(II) ion = Hg+2. You cannot simplify the “2.”) Constructing the Name of an Ionic Compound (given the formula of a compound) Metal ions often have more than one possible charge and the trick is deciding on the charge of a metal ion in a particular compound. (Assume that the negative ion has only one possible charge.) 1. If the first ion in the compound only has one possible charge, simply write the names of the ions one after another. (Omit the word “ion” from the names.) a. ZnI2: Zn+2 = zinc ion; I– = iodide ion and the name is zinc iodide. 2. If the first ion is a metal known to possess more than one possible charge OR a metal which is not on the table, “un-criss-cross” the subscripts and use them as charges. a. SnO2: un-criss-crossing the subscripts gives Sn 2 1 O -1 2 3. Next, if the known charge on the anion is double (or triple) that calculated by the un-crisscrossing process then double (or triple) the charges calculated for both the cations and anions. (This accounts for any subscripts that were divided by 2 or 3.) a. Since oxide ion is O–2, the charges are doubled to give Sn 4 1 O -2 2 4. Last, use Stock notation to write the cation’s name, followed by the anion’s name. a. Sn 4 1 O -2 2 is named tin(IV) oxide Example: Al2O3: This gives Al+3 and O–2. The aluminum ion only has one possible ionic charge. This is known as “aluminum oxide.” CaS: Calcium only has one possible ionic charge: +2. Sulfur is in the same group as oxygen, so it forms a –2 anion. The ionic compound is called “calcium sulfide.” FeCl2: Iron has more than one ionic charge, so when un-criss-crossing the subscripts, we get Fe2 1 Cl-1 2 . The charge on Cl is usually –1, so there is no need to double charges. The ionic compound is called “iron(II) chloride.” Ionic Bonding Ionic bond: Bond formed by the attraction of cations to anions. This bond is formed when one atom transfers electrons to another atom, so as to create one positive ion and one negative ion. Lewis structures can be used to represent the formation of ionic compounds. Example: This diagram shows how to use Lewis structures to explain Li and F combine to form Li+ and F– ions. The electrostatic attraction between the + and – charges hold the ions together. Example: BaCl2 is an ionic compound. Draw the Lewis structure for BaCl2. As seen from this diagram, ionic bonds will form when elements from opposite sides of the periodic table are combined. That is, when a metal and a non-metal are combined. Ionic bonds are quite strong. This is evidenced by examining the melting points of ionic compounds. Melting these ionic compounds would require lots of energy, the kind of energy that would be required to raise the temperature to these high levels. LiF = 845°C NaF = 993°C KCl = 770°C LiCl = 605°C Practice Problems: 1. Give the chemical formulae for the following ionic compounds: a) rubidium sulfide c) indium(III) telluride 2. Give the chemical name for the following ionic compounds: a) K2S b) MnO c) V2O5 d) PtCl4 3. Draw Lewis structures for the following ionic compounds: a) Na3P b) CaCl2









