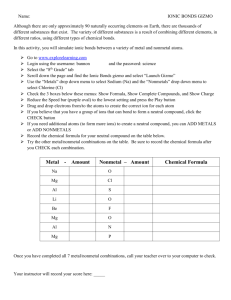
Grade 9 Chemistry Review: Periodic Table & Compounds
advertisement
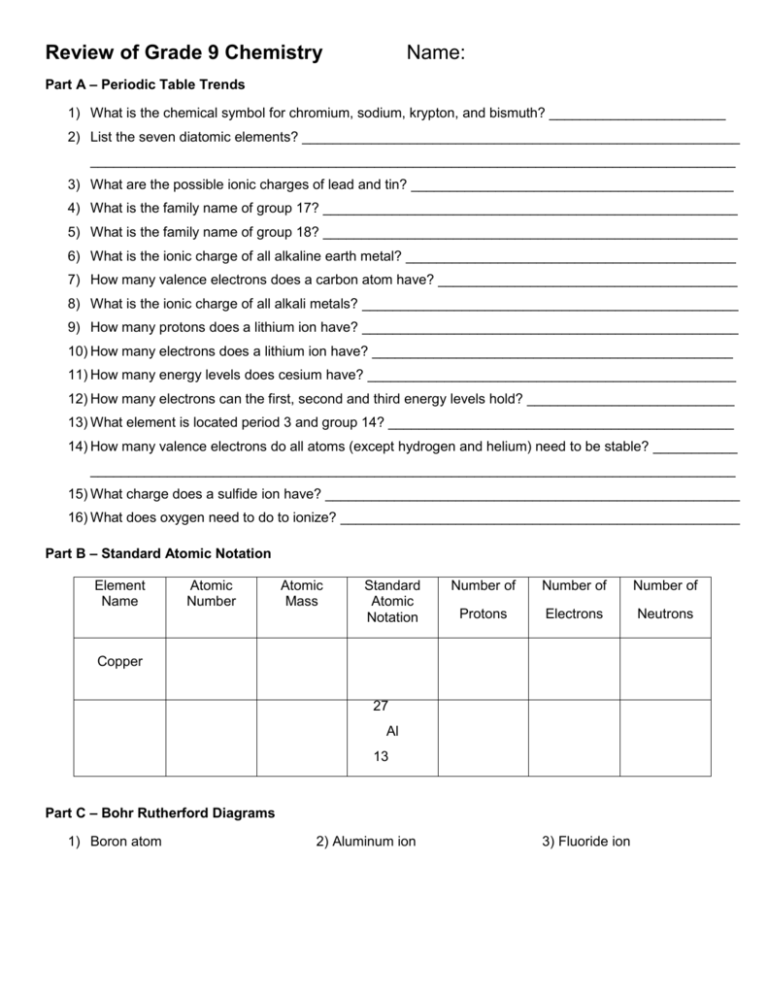
Review of Grade 9 Chemistry Name: Part A – Periodic Table Trends 1) What is the chemical symbol for chromium, sodium, krypton, and bismuth? _______________________ 2) List the seven diatomic elements? _________________________________________________________ ____________________________________________________________________________________ 3) What are the possible ionic charges of lead and tin? __________________________________________ 4) What is the family name of group 17? ______________________________________________________ 5) What is the family name of group 18? ______________________________________________________ 6) What is the ionic charge of all alkaline earth metal? ___________________________________________ 7) How many valence electrons does a carbon atom have? _______________________________________ 8) What is the ionic charge of all alkali metals? _________________________________________________ 9) How many protons does a lithium ion have? _________________________________________________ 10) How many electrons does a lithium ion have? _______________________________________________ 11) How many energy levels does cesium have? ________________________________________________ 12) How many electrons can the first, second and third energy levels hold? ___________________________ 13) What element is located period 3 and group 14? _____________________________________________ 14) How many valence electrons do all atoms (except hydrogen and helium) need to be stable? ___________ ____________________________________________________________________________________ 15) What charge does a sulfide ion have? ______________________________________________________ 16) What does oxygen need to do to ionize? ____________________________________________________ Part B – Standard Atomic Notation Element Name Atomic Number Atomic Mass Standard Atomic Notation Number of Number of Number of Protons Electrons Neutrons Copper 27 Al 13 Part C – Bohr Rutherford Diagrams 1) Boron atom 2) Aluminum ion 3) Fluoride ion Part D – Naming and Writing Chemical Compounds Give the compound name or formula as required for the following univalent ionic compounds. 1. Na2O 4. potassium chloride 2. MgBr2 5. magnesium fluoride 3. Be3N2 6. sodium sulfide Give the compound name or formula as required for the following multivalent ionic compounds. 1. PbCl4 4. iron (II) oxide 2. SnCl2 5. lead (II) nitride 3. CuF 6. tin (IV) bromide Give the compound name or formula as required for the following polyatomic ionic compounds. 1. MgCO3 4. lithium sulphate 2. Na2SO4 5. calcium hydroxide 3. K3PO4 6. lead (II) hydrogen carbonate Give the compound name or formula as required for the following molecular compounds. 1. SO2 4. NBr3 2. silicon tetrabromide 5. nitrogen monophosphide 3. dinitrogen pentoxide 6. CCl4 Part E - Bohr-Rutherford Diagrams 1) Draw the Bohr-Rutherford diagram for NITROGEN in the box provided. 2) Is nitrogen a metal, metalloid, or a non-metal? 3) How many valence electrons does nitrogen have? 4) How many inner electrons does nitrogen have? 5) When nitrogen ionizes what charge will it have? 6) What is the ion notation of the nitride ion?