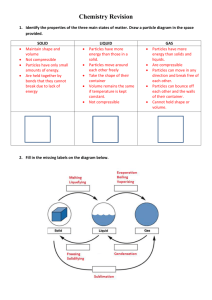
PREPARATION OF GASES
advertisement
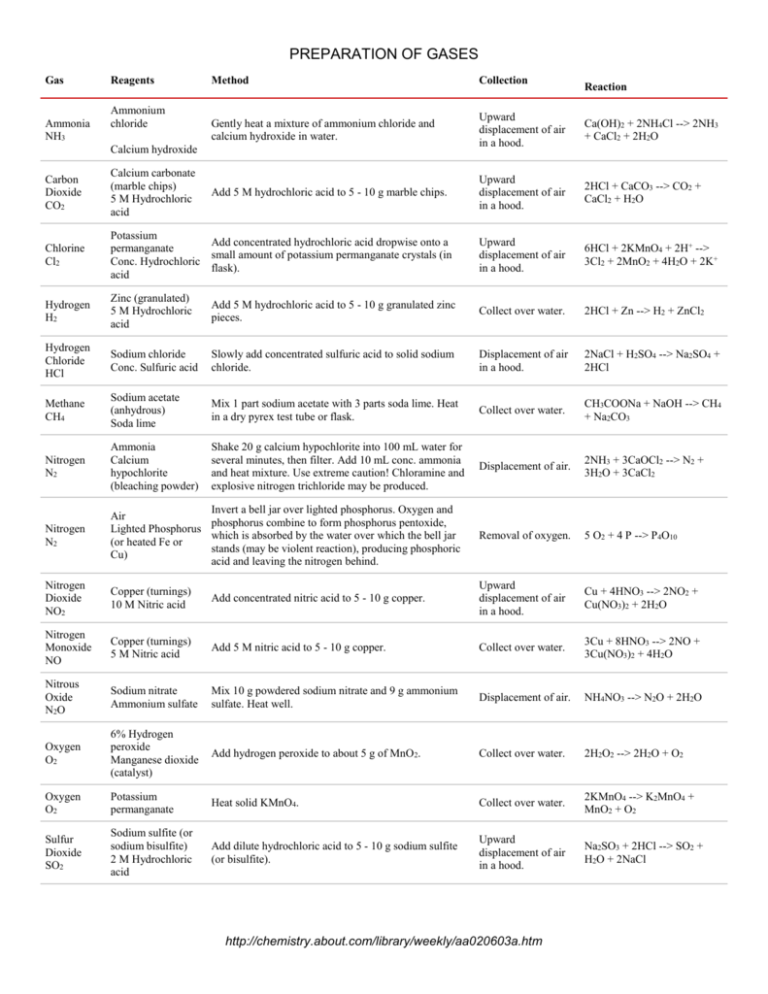
PREPARATION OF GASES Gas Ammonia NH3 Reagents Ammonium chloride Method Collection Gently heat a mixture of ammonium chloride and calcium hydroxide in water. Upward displacement of air in a hood. Ca(OH)2 + 2NH4Cl --> 2NH3 + CaCl2 + 2H2O Add 5 M hydrochloric acid to 5 - 10 g marble chips. Upward displacement of air in a hood. 2HCl + CaCO3 --> CO2 + CaCl2 + H2O Calcium hydroxide Reaction Carbon Dioxide CO2 Calcium carbonate (marble chips) 5 M Hydrochloric acid Chlorine Cl2 Potassium Add concentrated hydrochloric acid dropwise onto a permanganate small amount of potassium permanganate crystals (in Conc. Hydrochloric flask). acid Upward displacement of air in a hood. 6HCl + 2KMnO4 + 2H+ --> 3Cl2 + 2MnO2 + 4H2O + 2K+ Hydrogen H2 Zinc (granulated) 5 M Hydrochloric acid Add 5 M hydrochloric acid to 5 - 10 g granulated zinc pieces. Collect over water. 2HCl + Zn --> H2 + ZnCl2 Hydrogen Chloride HCl Sodium chloride Conc. Sulfuric acid Slowly add concentrated sulfuric acid to solid sodium chloride. Displacement of air in a hood. 2NaCl + H2SO4 --> Na2SO4 + 2HCl Methane CH4 Sodium acetate (anhydrous) Soda lime Mix 1 part sodium acetate with 3 parts soda lime. Heat in a dry pyrex test tube or flask. Collect over water. CH3COONa + NaOH --> CH4 + Na2CO3 Nitrogen N2 Ammonia Calcium hypochlorite (bleaching powder) Shake 20 g calcium hypochlorite into 100 mL water for several minutes, then filter. Add 10 mL conc. ammonia and heat mixture. Use extreme caution! Chloramine and explosive nitrogen trichloride may be produced. Displacement of air. 2NH3 + 3CaOCl2 --> N2 + 3H2O + 3CaCl2 Nitrogen N2 Invert a bell jar over lighted phosphorus. Oxygen and Air phosphorus combine to form phosphorus pentoxide, Lighted Phosphorus which is absorbed by the water over which the bell jar (or heated Fe or stands (may be violent reaction), producing phosphoric Cu) acid and leaving the nitrogen behind. Removal of oxygen. 5 O2 + 4 P --> P4O10 Nitrogen Dioxide NO2 Copper (turnings) 10 M Nitric acid Add concentrated nitric acid to 5 - 10 g copper. Upward displacement of air in a hood. Cu + 4HNO3 --> 2NO2 + Cu(NO3)2 + 2H2O Nitrogen Monoxide NO Copper (turnings) 5 M Nitric acid Add 5 M nitric acid to 5 - 10 g copper. Collect over water. 3Cu + 8HNO3 --> 2NO + 3Cu(NO3)2 + 4H2O Nitrous Oxide N2O Sodium nitrate Ammonium sulfate Mix 10 g powdered sodium nitrate and 9 g ammonium sulfate. Heat well. Displacement of air. NH4NO3 --> N2O + 2H2O Oxygen O2 6% Hydrogen peroxide Manganese dioxide (catalyst) Add hydrogen peroxide to about 5 g of MnO2. Collect over water. 2H2O2 --> 2H2O + O2 Oxygen O2 Potassium permanganate Heat solid KMnO4. Collect over water. 2KMnO4 --> K2MnO4 + MnO2 + O2 Sulfur Dioxide SO2 Sodium sulfite (or sodium bisulfite) 2 M Hydrochloric acid Add dilute hydrochloric acid to 5 - 10 g sodium sulfite (or bisulfite). Upward displacement of air in a hood. Na2SO3 + 2HCl --> SO2 + H2O + 2NaCl http://chemistry.about.com/library/weekly/aa020603a.htm