Chemistry Revision
advertisement
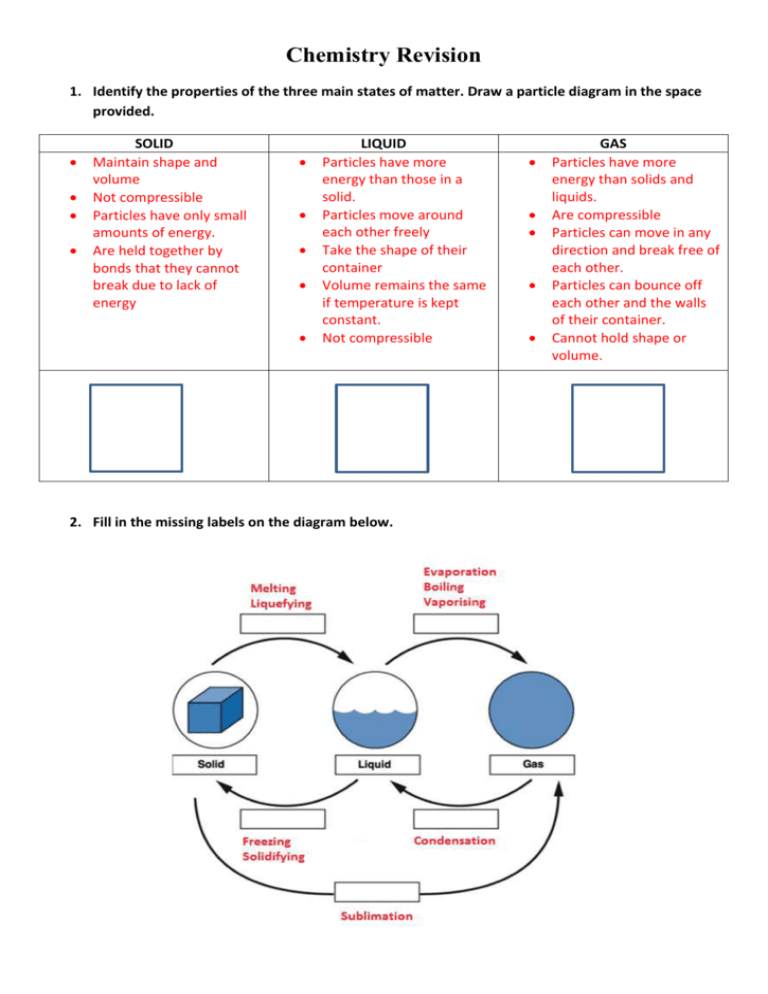
Chemistry Revision 1. Identify the properties of the three main states of matter. Draw a particle diagram in the space provided. SOLID Maintain shape and volume Not compressible Particles have only small amounts of energy. Are held together by bonds that they cannot break due to lack of energy LIQUID Particles have more energy than those in a solid. Particles move around each other freely Take the shape of their container Volume remains the same if temperature is kept constant. Not compressible 2. Fill in the missing labels on the diagram below. GAS Particles have more energy than solids and liquids. Are compressible Particles can move in any direction and break free of each other. Particles can bounce off each other and the walls of their container. Cannot hold shape or volume. 3. Define the following terms: a) Deposition: Process in which a gas turns into a solid without going through a liquid phase. b) Expansion: Increase in volume of a material due to the addition of energy. For example when a metal is heated, the particles become “excited” and the metal expands as the particles have increased movement. Think back to the “Balloon Expansion” video we watched. c) Contraction: Decrease in volume of a material in response to a reduction in energy. 4. Explain the difference between physical and chemical change. Give one example of each kind of change. Physical changes alter the physical properties of a material but do not change the chemical make-up. For example if you cut a piece of paper in half, you may have changed its shape and size but it is still paper. Chemical changes result in new substances being formed. For example when you combine bicard soda and vinegar, you can observe bubbling/fizzing which indicates a gas is being produced. 5. A ______SOLUTE__________ and a ________SOLVENT________ combine to form a solution. 6. True or False? Salt dissolving in water is an example of a chemical change. FALSE it is a physical change. The salt particles have just been broken down into such small pieces that we can no longer see them. If we evaporated the water, the salt crystals would reform. 7. Define the following terms: a) Endothermic reaction: A reaction that absorbs heat energy from its surroundings. b) Exothermic reaction: A reaction that produces heat energy. 8. Calculate the number of protons, neutrons and electrons in the following atoms. Atomic Symbol Number of Protons Number of Neutrons Number of Electrons 16 8O 8 8 8 23 11Na 11 12 11 19 20 19 9. Give two reasons why the air in a basketball is considered matter. i) It takes up space ii) It has mass 10. What is the difference between corrosion and rusting? Corrosion refers to a metal reacting with oxygen. Rusting refers specifically to the corrosion of iron. 11. Circle the reactants and underline the products in the chemical equation given below. Word equation: Acetic acid + Sodium bicarbonate ------> Sodium acetate + Water + Carbon dioxide Formula equation: C2H4O2 + NaHCO3 ------> NaC2H3O2 + H2O + CO2 12. When sodium chloride (NaCl) and silver nitrate (AgNO 3) are combined they produce silver chloride (AgCl) and sodium nitrate(NaNO3). Write this reaction as a WORD EQUATION Sodium chloride + silver nitrate -----> silver chloride + sodium nitrate Write this reaction as a FORMULA EQUATION NaCl + AgNO3 ----> AgCl + NaNO3 13. Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water (H2O). Write this reaction as a WORD EQUATION Hydrochloric acid + sodium hydroxide ----> sodium chloride + water Write this reaction as a FORMULA EQUATION HCl + NaOH ----> NaCl + H2O 14. What is a chemical reaction? A chemical reaction is when atoms rearrange to form new substances. 15. State the formula to work out density? Density = volume/mass 16. For each of the following say whether it is an element, compound or mixture: Water Compound Carbon Element Sugar in water Mixture Salt in water Mixture Iron Element Hydrochloric acid Compound Lemonade Mixture C12H22O11 Compound











