Physical and Chemical Properties and Changes Lab_Student Copy
advertisement

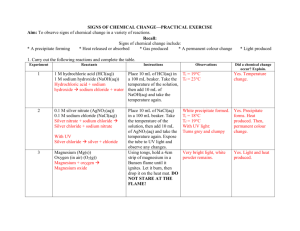
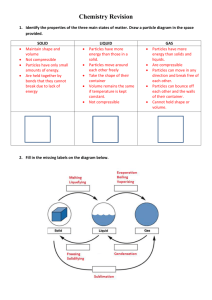
Name _____________________________________________ Class ________ Date _____________ Physical and Chemical Properties and Changes This laboratory activity will allow the students to: 1. write their own laboratory experiment paper; 2. observe physical and chemical properties; 3. observe physical and chemical changes; 4. identify evidences of chemical change; 5. differentiate a. physical and chemical properties; b. physical and chemical changes; and c. intensive and extensive properties; 6. write formulas of chemicals used; and 7. write chemical equations to represent chemical changes. Part 1. Prelab Questions: 1. When making observations in a chemistry laboratory class, certain physical properties of objects (or chemicals) are described. List ten (10) different kinds of physical properties that you have learned. a. _________________________________ f. ______________________________________ b. _________________________________ g. ______________________________________ c. _________________________________ h. ______________________________________ d. _________________________________ i. ______________________________________ e. _________________________________ j. _______________________________________ 2. What is a physical change? 3. What is a chemical property? 4. How will you be able to observe chemical property? 5. What is a chemical change? 6. What are some “evidences” that are observed when a chemical change happens? a. ______________________________________________________________________ b. ______________________________________________________________________ c. ______________________________________________________________________ d. ______________________________________________________________________ e. ______________________________________________________________________ f. ______________________________________________________________________ g. ______________________________________________________________________ 7. In your own words, what is the difference between a chemical change and a physical change? 8. Identify each of the following as a physical or a chemical change (write P for physical change and C for chemical change) and explain why each is a chemical or physical change. _____ a. You leave your bicycle out in the rain and it rusts. _____ b. A sugar cube dissolves in water. _____ c. Pure sodium is normally stored in oil. When the oil is removed, a flame is produced because the potassium can now react with oxygen in the air. _____ d. Sand is mixed with water. _____ e. Scientists break up water into H2 and O2 (this process is called hydrolysis). _____ f. You are cleaning your bathroom and you accidentally mix bleach and ammonia, which produces a toxic gas, which makes you pass out. _____ g. Burning coal for a barbecue. _____ h. Chewing up a bite of hamburger. _____ i. You take a shower and your wet hair begins to dry. _____ j. Silver nitrate and sodium chloride mix to form a grey-violet precipitate. _____ k. Trimming a plant because it has grown too high. _____ l. Mashing up potatoes to make mashed potatoes. _____ m. Sodium polyacrylate and water mix in a bowl to form a gel. When the water evaporates, the sodium polyacrylate remains in the bowl. _____ n. Hydrogen peroxide is poured on some liver and the liver begins to break down. 9. Write your own example, identify it as a chemical or physical change and explain. 10. Identify each statement as being true or false. Write TRUE if the statement is true and write FALSE if the statement is false. _________ A change in size or shape is a physical change. _________ A chemical change means a new substance with new properties was formed. _________ An example of a chemical change is when water freezes. _________ When platinum is heated, then cooled to its original state, we say this is a physical change. _________ When milk turns sour, this is a physical change because a change in odor does not indicate a chemical change. _________ When magnesium is burned, ash forms. We say this is a physical change because the magnesium looks different. _________ When citric acid and baking soda mix, carbon dioxide is produced and the temperature decreases. This must be a chemical change. Part 2. Writing the lab. a. Write a laboratory procedure that will allow you to observe physical properties, chemical properties, physical changes, and chemical changes of the following chemicals: 1. Magnesium and hydrochloric acid 8. Sucrose and oxygen 2. Silver nitrate and hydrochloric acid 9. Potassium iodide and lead nitrate 3. Copper (II) chloride and aluminum foil 10. Sulfuric acid and antacid 4. Sodium bicarbonate and water 11. Powdered milk, sucrose and water 5. Sodium bicarbonate and acetic acid 12. Iodine and hot air (and cold air) 6. Ammonium chloride and water 13. Sodium chloride and water 7. Sodium hydroxide and water b. Prepare a data table where you will write your results/observations for the experiment that you just wrote. Part 3. Laboratory Experiment. After your lab procedure is approved by your instructor, perform your lab experiment. Part 4. Data Analysis.