ps700-coll2-hayden
advertisement
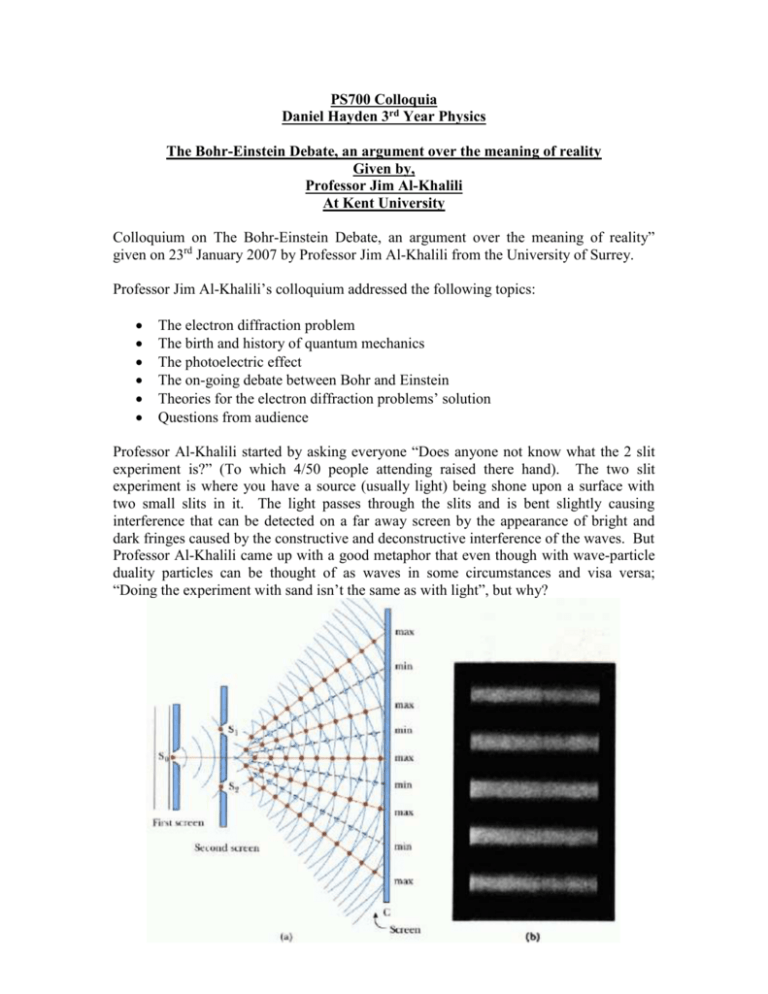
PS700 Colloquia Daniel Hayden 3rd Year Physics The Bohr-Einstein Debate, an argument over the meaning of reality Given by, Professor Jim Al-Khalili At Kent University Colloquium on The Bohr-Einstein Debate, an argument over the meaning of reality” given on 23rd January 2007 by Professor Jim Al-Khalili from the University of Surrey. Professor Jim Al-Khalili’s colloquium addressed the following topics: The electron diffraction problem The birth and history of quantum mechanics The photoelectric effect The on-going debate between Bohr and Einstein Theories for the electron diffraction problems’ solution Questions from audience Professor Al-Khalili started by asking everyone “Does anyone not know what the 2 slit experiment is?” (To which 4/50 people attending raised there hand). The two slit experiment is where you have a source (usually light) being shone upon a surface with two small slits in it. The light passes through the slits and is bent slightly causing interference that can be detected on a far away screen by the appearance of bright and dark fringes caused by the constructive and deconstructive interference of the waves. But Professor Al-Khalili came up with a good metaphor that even though with wave-particle duality particles can be thought of as waves in some circumstances and visa versa; “Doing the experiment with sand isn’t the same as with light”, but why? Quantum mechanics describes the way that subatomic and very small objects behave but the same experiment with small particles yields a different result. He suggested you could try to fool the experiment by letting one electron through at a time, and as you start to do so the pattern does indeed appear to be random. But as you carry on you gradually begin to see interference patterns building up again. The question raised by this paradox was does the electron split in two and spread out like a wave? Half detected through one slit and half through the other. Scientists set up an experiment with a detector over one slit, fired a certain number of electrons and saw that only half went through the slit they were observing and so obviously the other half were going through the other slit. But when they looked at the diffraction pattern, no interference pattern was seen, it was as if they were now acting as particles. Turn the detector off and the interference pattern would appear again, no one knows why this is so but Professor Al-Khalili stressed that “This isn’t theory, this actually happens”. The birth of Quantum mechanics was around 1900-1905 with a man called Max Planck, helped by the work of L.Boltzmann. Before them, professor Al-Khalili explained, people used to think objects were infinitely divisible, but Boltzmann was the first to say that there must be a small indivisible “something”. Planck said that heat radiation given off by bodies comes in lumps or “quanta” and then Einstein came in and said actually all electromagnetic radiation comes in lumps. Einstein actually won the Nobel Prize for this using his photoelectric effect experiment. The photoelectric effect is a quantum phenomenon where incoming electromagnetic radiation of high enough energy can impart this energy to the electrons within a metal giving them enough kinetic energy to overcome the binding energy and leave the metal. It used to be thought that the higher the energy of the photons the more electrons would be released but this experiment showed that in fact it depended on the frequency of the photons, not the intensity, i.e. photons inbound with enough energy to electrons released was 1-1 thus electromagnetic radiation must come in discrete packets. Niels Bohr was working on quantum mechanics in an era where it was already a well established theory (1924-26). Professor Al-Khalili actually compared Bohrs’ genius to that of Einstein’s but said that “Bohr was however a very bad talker, not a very good lecturer and although a good footballer, not very good at getting his point heard”. A disciple of Bohr was Werner Heisenberg and although they fell out in the Second World War Heisenberg was “one of the genius’s in the world” because he said that in quantum mechanics properties don’t even exist until you measure them. W.Pauli, he said, also deserved a mention for having a part in the success of quantum mechanics and is most famous for his exclusion principle stating that no two electrons in an atom can be at in the same state or configuration at any point in time, this laid the foundation for the whole of modern chemistry. Professor Jim Al-Khalili mentioned Einstein’s list of achievements but said that with time he become almost old hat, “The longer you’re in a subject the harder it is to break free”. The others were younger and now more revolutionary than Einstein so when all the best physicists in the world descended upon Copenhagen as they did annually that year to work on quantum mechanics, sparks were bound to fly. Professor Al-Khalili started then to talk about the friendship that Einstein and Bose had, they would walk around for hours at the conferences and debate on the meaning of quantum mechanics and associated experiments like that of Stern Gerlach and Compton. Of all the Physicists that went to the Solvay conference of Brussels in October 1927, two main groups emerged. One consisting of people like Einstein, Pauli, and Schrodinger who believed that the world was ultimately deterministic “God does not play dice” and the other with Bohr at the helm saying that basically he did. At the time Einstein’s clan was not as good at attacking the Copenhagen groups’ ideas and so Bohr won through (As an aside Al-Khalili mentioned that apparently Dirac was above it all). This is where the famous debate between Einstein and Bohr started, constantly going back and forth with letters and meetings every so often where Einstein would think he had something to prove Bohr wrong and Bohr disproving it so Einstein would then go off again coming back next time with something else only to be disproved again, all the time with the thought experiments becoming more and more sophisticated and complex trying to prove that quantum mechanics was false (or at least incomplete). But at the 6th Solvay conference in 1930, Einstein was convinced he had proof. Bohr was worried because Einstein seemed supremely confident in his new idea to disprove quantum mechanics and it did indeed cause Bohr some problems as shown below. Einstein thought of a box that containing electromagnetic radiation and a clock that controlled the opening of a shutter which covers a hole made in one of the walls of the box. The shutter uncovers the hole for a time Δt which could be chosen arbitrarily. During the opening, theoretically a photon would escape through the hole. In this way a wave of limited spatial extension could be created. In order to challenge the indeterminacy relation between time and energy that Bohr had always championed from quantum mechanics, it was necessary to find a way to determine with an adequate precision the energy that the photon has brought with it. At this point, Einstein turned to his celebrated relation between mass and energy of special relativity: . From this it follows that knowing the mass of an object provides a precise knowledge about its energy. The argument is therefore very simple, if one weighs the box before and after the opening of the shutter and if a certain amount of energy has escaped from the box, the box will be lighter. The change in mass multiplied by will provide precise knowledge of the energy emitted. Also the clock would indicate the precise time the particle left the box. Since in principle, the mass of the box can be determined to an arbitrary degree of accuracy, the energy emitted can be determined with a precision ΔE as accurately as one wanted. Therefore, the product ΔEΔt can be rendered less than what is implied by the principle of indeterminacy. But by the next morning Bohr had worked it out and worse still by again showing that Einstein's subtle argument was not conclusive, using one of Einstein’s own great ideas: The theory of relativity. Bohr showed that, in order for Einstein's experiment to work, the box would have to be suspended on a spring in the middle of a gravitational field. In order to obtain a measurement of weight, a pointer would have to be attached to the box which corresponded with the index on a scale. After the release of a photon, weights could be added to the box to restore it to its original position and this would allow us to determine the weight. But in order to return the box to its original position, the box itself would have to be measured. The inevitable uncertainty of the position of the box translates into an uncertainty in the position of the pointer and of the determination of weight and therefore of energy. On the other hand, since the system is immersed in a gravitational field which varies with the position, according to the principle of equivalence the uncertainty in the position of the clock implies an uncertainty with respect to its measurement of time and therefore of the value of the interval Δt. A precise evaluation of this effect leads to the conclusion that the relation , cannot be violated (Heisenberg Uncertainty principle). Bohr and the Copenhagen group had one the debate. The Copenhagen interpretation of the electron diffraction experiment is basically that the act of looking affects the experiment, “We can never peak behind the curtain”. Quantum mechanics shows you the answer and predicts what is seen but as soon as you look it returns to what you would expect classically. Bohr said there was no point in looking because to check is to change the outcome so you can’t win and quantum mechanics underlies nearly everything we see around us. Professor Al-Khalili then went on to explain other interpretations that have been hypothesized over the years like a rather good one called the Bohm interpretation where all the electrons are considered point-like particles that occupy precisely defined regions of space at all times. When one performs a double slit experiment we want to know the positions on a screen at which the electrons arrive individually, one at a time. Over time, the positions at which the electrons are detected build up a pattern characteristic of wave interference. The usual Copenhagen interpretation is puzzling in that a single entity, the electron, is said to exhibit characteristics of both particle and wave. The Bohm interpretation accounts for the same phenomena by saying that both a particle and a wave do exist. The particle aspect is present because each electron traverses one slit or another, but never both. The wave aspect is present because the electron's pilot wave traverses both slits. Also there is the Transactional interpretation which is slightly more “out there” but basically says that a signal is somehow sent back in time from when the electrons have already arrived at the screen, telling the electrons where to go. This is however a rather ambitious theory as it is almost a paradox fore the signal to be sent back there would have to theoretically be an electron that has already traveled there at some time for the first time and therefore the problem is still unexplained as to why the electron took that route in the first place. However another good theory is the Many-world or Multi-verse theory in which there are actually infinitely many universes stacked on top of each other and at every decision the universe splits again, therefore we are just taking the specific route through the universes that we are experiencing. Lastly there was the Sum over histories interpretation which is likely one of the most well known of those mentioned. In this theory an atom explores every possible path simultaneously, but all summed together cancel leaving just one, which we see. Professor Al-Khalili then went on to describe entanglement which is the apparent link between two originally connected particles even when they are an arbitrary distance apart. The EPR paradox of 1935 (Einstein, Podolsky, Rosen) said two particles leaving back to back are quantum waves until measured. When measured greater than the speed of light apart, measuring one defines the other instantaneously. But Bell’s theorem in its simplest form says that “No physical theory of local hidden variables can ever reproduce all of the predictions of quantum mechanics” and an experiment was thought of to see if two particles were obviously one and the other because they were once together or as quantum mechanics said, really were only defined when measured. This was Alain Aspects’ experiment and it showed that Einstein was wrong, however illogical quantum mechanics seemed it was correct and particles did indeed seem to be linked more than could be accounted for. Many physicists have views on these conclusions; Mermin said that despite this the explanation of the phenomenon is still incomplete (Which Professor Jim Al-Khalili agreed with), Cushing claimed that the Copenhagen group just got their idea out there first and that it is by far not the best explanation and lastly Gellmann claimed Bohr had brainwashed the people around him into believing it was solved when really it never was. Professor Al-Khalili finished by posing the audience a question; “Is the difference just philosophical?” and that Bohr said it was wrong to think that physicists were there to find out how nature is but merely too find out what we can about nature. (The talk over ran by 15 minutes) Questions: Q: Does string theory come into this? A: Yes, QM explains small things, Newtonian explains macroscopic things, Physicists want to explain everything and string theory is a theory that joins QM with special relativity. Q: Is the next step to think of better experiments? A: Yes, we are all the time thinking of experiments, some better than Einstein or Bohr could have ever dreamed of, Quantum optics is a whole field doing just that. Q: Can macroscopic things show quantum behaviour? A: Yes, but still unknown where Macroscopic ends and the quantum world begins. Talked about quantum de-coherence. Q: Is Planck’s constant always the same value? A: Not necessarily Q: Does it matter? A: Yes Q: What would happen with the double slit experiment if you used marbles? A: They are using bigger things all the time to do the experiment, Bucky balls (Carbon 60), and even possibly small viruses in the future and it still works, but eventually there will come a limit. Marbles are too big. Strengths Professor Jim Al-Khalili was very clear and well spoken, getting his message across well. The main thing that impressed me was the quality of diagrams to aid the audience as he went through his talk, every slide had something to help you better understand what he was talking about and I felt that lay and scientists alike could enjoy what they were seeing. His talk was vibrant and passionate, one clearly being able to see his own interest in the subject, but keeping it light and airy rather than serious (like a lecture). His language flowed well and every subject he talked about was explained thoroughly so again everyone was able to understand. I learnt a lot myself about the electron diffraction problem and understood the many theories that try to answer it a lot better after attending the talk. I was surprised and delighted to hear all about the annual Solvay conferences and to see a picture of many of the best scientists that have ever lived sitting together in one picture, it even sent a small shiver down my spine and a thought of jealousy that I could not be there at that time to hear them all, it must have been awe inspiring. The power point presentation was colourful and eye catching with the pace through the slides being about right and able to read everything that was on each of the slides easily before moving on to the next, basically a very well matched visual to oral presentation of the ideas. Professor Al-Khalili seemed supremely confident throughout and answered all questions at the end with accuracy and open mindedness even in the face of questions that to a scientist may have at times seemed obvious. Weaknesses Professor Al-Khalili did over run the hour allotted by 15 minutes, yet still it felt like the last 10 minutes of the talk were a little rushed (As If there was simply too much to fit in the hour allowed). There were a couple of spelling mistakes on the slides but generally not too bad. There was a tendency to do asides a little too often, it is cute but relatively un-important to know that Niels Bohr was the sub goal keeper for his national side and these facts a little too often felt a bit too off topic (especially as he over ran by 15minutes). I would personally have cut out so many of the unimportant asides and concentrated on the topic more, still giving time to the relevant asides but keeping them as asides rather than small sections of the talk. It surprised me in a bad way that sometimes he seemed to skip back slides and then forward again to explain certain aspects rather than make the presentation slides flow better, his speech and personal presentation flowed very well with the slides and it was just a shame that although visually and factually stunning his power point presentation was not as linear as it could have been. Also there was a tendency to skip large periods of time from the birth of quantum mechanics 1900-1905 straight to 1924-26 when it was already a well established theory. This is fine for scientists who understand that things went on in between but to lay it might have seemed that suddenly in 1924 it was accepted and started moving forwards again when in fact work has never and probably will never stop on quantum mechanics, and it is only the subsequent work in the many intervening years between breakthroughs that lead to the next.








