Discovery of the Electron, Models & Theories
advertisement

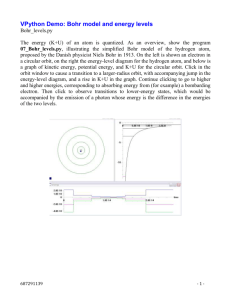
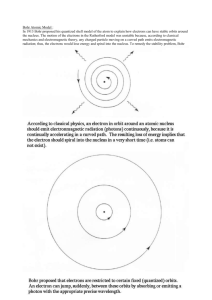
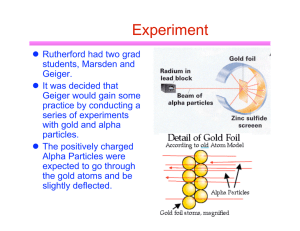
Discovery of the Electron, Models & Theories Jeffrey Thorin Daniel JJ Thompson Robert Millikan • Discovered the electron • Wrote books on atomic theory and structure • First to suggest that atoms have parts • Found the accurate way to decide the charge carried with an atom Niels Bohr • Helped create a picture of the atomic structure(Bohr model) • This said that electrons orbit the nucleus in fixed circular orbits Louis de Broglie • Looked into Bohr’s investigation and thought electrons moved in waves rather that circular orbits Werner Heisenberg • Known for his theory in quantum mechanics • Proved Bohr’s theory wrong about the movement of electrons • Said we can’t assign to an electron a position in space at a given time, nor follow it in its orbit Erwin Schrodinger • Developed a waveequation that accurately gave energy levels to atoms Bohr Model An atom consisting of small positively charged nucleus orbited by negatively charged electrons Similar to planetary model where planets orbit the Sun Solar system uses gravitational forces whereas an atom uses electrostatic forces Electrons have certain orbits with certain radii Orbits are circular according to quantized energy levels Orbits with larger radii have larger energies Quantum Model Based on quantum theory, which says matter also has properties associated with waves Impossible to know the exact position and momentum of an electron at the same time Uses complex shapes of orbitals (electron clouds) volumes of space in which there is likely to be an electron Based on probability rather than certainty Experiments Thompson experimented with cathode rays and magnets that led to the discovery of the electron Millikan devised an oil-drop experiment where he’d drop tiny charged droplets to find out their specific charge Bohr based his model on experiments done by Ernest Rutherford; he saw Rutherford’s model wasn’t quite right and changed it Broglie saw that Bohr’s model, specifically the electrons, move in waves, not in a circular orbit Heisenberg also went off of Bohr’s model and elaborated more into quantum mechanics to explain the wave motion of electrons instead of a circular orbit Schrodinger used mathematical equations to predict the likelihood of finding the electron and eventually found one that worked Time Line of History Scientists from the early 1900s Theories developed without use of more precise modern technology Over time… Bohr’s model had a lot of problems and inaccuracies to it Quantum model is considered “standard” and is also considered the most accurate physical theory ever created