Chem 30 Solubility - FW Johnson Collegiate
advertisement

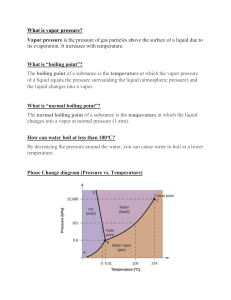
Liquid Solutions: Miscible – liquids that dissolve in each other: water & food colouring, water & alcohol Immiscible – Liquids that don’t dissolve together (when you look at them, you can see layers): Oil & Water Types of Solutions: Unsaturated - all solute dissolved o o excess solvent rate of dissolving > rate of solid reforming Saturated - not all of the solute is dissolved o o o Rate of dissolving of solute is balanced by rate of it coming out of solution Excess solute Equilibrium established If you DISTURB the equilibrium (ex. Heat solution), you can increase solubility of solute Supersaturated – more solute dissolved than normal -Unstable - Few examples - Solute dissolved in solvent - Rate of solid formation > rate of dissolving when a “seed” crystal is added