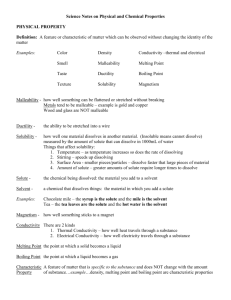
Solubility in Solutions
advertisement

Solubility in Solutions Honors Chemistry Complete each question is as much detail as possible. 1) What does it mean to say “likes dissolve likes?” 2) Why don’t oil and vinegar mix? Answer in terms of the molecules of the two liquids. 3) Indicate the solvent that will best dissolve the solute. Use molecular expliantions to explain your choices: a. Solute: Lithium Acetate Solvents: carbon disulfide, Ch2Cl2 b. Solute: Boron trichloride Solvents: carbon tetrachloride, water c. Solute: Phosphorus triiodide Solvents: ammonia, water 4) Explain which process would occur most quickly, a polar solvent dissolving a polar solute or a nonpolar solvent dissolving a nonpolar solute. 5) Give an example of two liquids that are immiscible. Give an example of two liquids that are miscible.