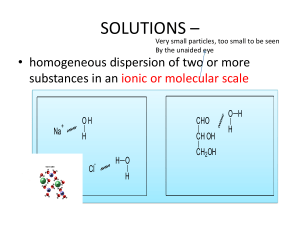
Grade 12 Core Science Quiz (1) LC2 Chapter 23
advertisement
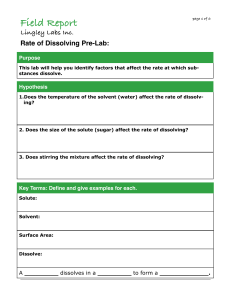
Class Work sheet# 7 Chapter (22) Section (1) Subject Name Date Grade 9 Physical Science Cluster QI. Choose the best answer: 1. In a solution, the substance that is being dissolved is the _____. a. solute b. solvent c. liquid d. gas 2. The air that you breathe is an example of a(n) _____ solution. a. gaseous b. solid c. liquid d. amalgam 3. An alloy is an example of a _____ solution. a. gaseous b. liquid c. solid d. dilute 4. . Increasing the surface area of a solid _____. a. slows the speed of dissolving b. has no effect on the speed of dissolving c. increases the speed of dissolving d. causes the solid to ionize 5. what can increase to make a gas more soluble in a liquid? a. particle size b. stirring c. pressure d. temperature QII. Answer the following questions: 1- What is the effect of increasing temperature on the rate of dissolving? ___________________________________________________________________________________________________________________ _________________________________________________________________________________________________________________. 2- Describe how the metal atoms in an alloy are mixed? ___________________________________________________________________________________________________________________ ___________________________________________________________________________________________________________________ 3- Why does stirring increase the rate at which a solute dissolves into a solvent? ___________________________________________________________________________________________________________________ ___________________________________________________________________________________________________________________ 4- How does crystal size affect the rate at which solute can be dissolved? ___________________________________________________________________________________________________________________ ___________________________________________________________________________________________________________________ Page 1 of 2 QIII. Directions: Complete the table below by writing the missing information in the appropriate box. Then answer the question below. Solution type Solvent Solute Example 1. gas gas 2. solid 3. solid salt water dental amalgam 4. liquid 5. liquid 6. solid club soda Liquid brass Study the information in your table carefully. What is true about the state of the solvent and the type of solution produced? ___________________________________________________________________________________________________________________________ ___________________________________________________________________________________________________________________________ __________________________________________________________________________________________________________________________. QIV. Directions: Circle the term in parentheses that makes each statement true. 1. When a solid is being dissolved in a liquid, stirring (speeds up, slows down) the dissolving process. 2. A gas dissolves faster in a liquid if the temperature of the liquid is (increased, decreased). 3. A gas’s solubility is faster in a liquid when under (high, low) pressure. 4. By stirring a gas in a liquid, its solubility (speeds up, slows down) the dissolving process. 5. A solid dissolves faster in a liquid if the temperature of the liquid is (increased, decreased). 6. The (larger, smaller) the surface area of a solid, the faster it will dissolve. Page 2 of 2