Name: Photoelectron Spectroscopy Worksheet 1. PES experiments
advertisement

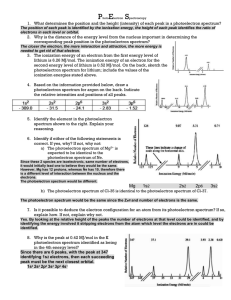
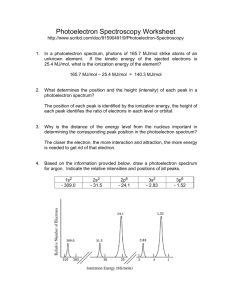
Name: __________________________ Photoelectron Spectroscopy Worksheet 1. PES experiments can use an X-ray wavelength of 0.8721 nm. Calculate the energy of the X-ray photon used in the PES experiment described. Use the equations given on you AP equation sheet. 2. The photon in question 1 strikes atoms of an unknown element. The kinetic energy of the ejected electrons is 2.627 × 10−16 J, what is the ionization energy of the element? 3. What determines the position and the height (intensity) of each peak in a photoelectron spectrum? 4. Why is the distance of the energy level from the nucleus important in determining the corresponding peak position in the photoelectron spectrum? 5. Based on the information provided below, draw a photoelectron spectrum for argon. Indicate the relative intensities and positions of all peaks. 1s2 - 309.0 2s2 - 31.5 2p6 - 24.1 3s2 - 2.83 3p6 - 1.52 6. Is it possible to deduce the electron configuration for an atom from its photoelectron spectrum? If so, explain how. If not, explain why not. 7. Identify the element in the photoelectron spectrum shown below and write its electron configuration. 8. Identify if either of the following statements is correct. Briefly explain your reasoning: 2+ is expected to be identical to the 35Cl is identical to the photoelectron a) The photoelectron spectrum of Mg photoelectron spectrum of Ne. b) The photoelectron spectrum of spectrum of 37Cl.