Electrolysis of Water: Semester 1 Prelab (Day 1)
advertisement

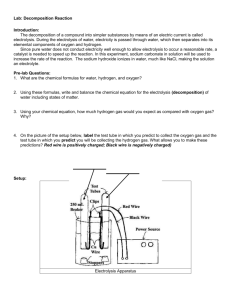
Name: _________________________________ Lab Report Due Date: ___________________ EPS/Biology Integrated Mrs. Dion and Mr. Hartshorn Electrolysis of Water: Semester 1 Prelab (Day 1) Background: The decomposition of a compound into simpler substances by means of an electric current is called electrolysis. During the electrolysis of water, electricity is passed through water, which then separated into its elemental components of oxygen (O2 gas) and hydrogen (H2 gas). Since pure water does not conduct electricity well enough to allow electrolysis to occur at a reasonable rate, a catalyst is needed to speed up the reaction. In this experiment, sodium carbonate (Na2CO3) in solution will be used to increase the rate of the reaction. The sodium carbonate ionizes in water, much like NaCl, making the solution an electrolyte. The decomposition of water into hydrogen and oxygen gas demonstrates the conservation of mass. Please take a minute to define the words in bold in the paragraph above so that you have a better understanding of this lab. Decomposition- Electrolysis- Conduct- Catalysis- Conservation of Mass- Problem: How much of each gas, Hydrogen and Oxygen, will be collected when water is broken down by electrolysis? Hypothesis: Based on the chemical formula of water, _____________ parts of Hydrogen gas will be collected for every ____________________ parts of Oxygen gas that will be collected. This will be the case because of the law of conservation of mass which states: ___________________________________________________________________________________ The gases will be detected because Hydrogen gas has the chemical property of (page 461) ________________________________ and Oxygen has the chemical property of (page 641) _______________________. Therefore, when flame is applied to Hydrogen it will___________________ and when glowing embers applied to Oxygen, it will ___________________________________. Name: _________________________________ Lab Report Due Date: ___________________ EPS/Biology Integrated Mrs. Dion and Mr. Hartshorn Electrolysis of Water: Semester 1 Laboratory (Day 2) Materials: 2 small test tubes 2 small corks 2 small clamps 2 rubber bands 2 J-hook electrodes 1 pegboard 1 Sharpie marker 1 600-mL beaker ½ full with water 5 mL Sodium Carbonate 2 12-volt batteries 3 wires with alligator clips 2 splints 1 graduated cylinder Procedure: Look at page 642, figure 11 as a reference. Please check off each step as you work. 1) □ Safety: Put on goggles. 2) □ Attach the two small clamps onto the pegboard. Position them over the 600mL beaker of water level with the rim of the beaker. 3) □ Fill the two small test tubes with some of the water. Using your thumb or the corks to seal the tip of the test tube, invert it under the water. Do not leave any air bubbles. 4) □ Insert the “J” part of the J-hooks under each test tube beneath the water. Secure them at the top with rubber bands. Clamp the test tubes in place. 5) □ Lower the J-hooks so that electricity can be conducted through the water and not be impaired by the glass of the test tubes. Be sure the electrodes are positioned so that the bubbles will float up and into the tubes. 6) □ Connect your batteries into a series circuit. There should be a complete “circle” of electricity with the water making the connection in the last bit of the circle. Remember that positive poles always connect to negative poles and vice versa. 7) □ Make observations of any bubble formation at each electrode. 8) □ Add 5mL of Sodium Carbonate to the water. 9) □ Make observations of any difference in the rate of bubble formation. 10) □ After 20 minutes, record the level of gas in each test tube in data table. One member of the group put on latex gloves and disconnect the battery and test the gases for flammability: Oxygen=right side up, glowing splint; Hydrogen=upside down, flaming splint. 11) □ Clean up apparatus. *During the lab, you need to make sure that you sketch the design set-up and fully label it. This is part of the final lab report. The next page gives you a place to do this work and a place to discuss the purpose of use for each piece of equipment. Name: _________________________________ Lab Report Due Date: ___________________ Design Set-up: Purpose for use: Test tubes: Beaker: J-electrode: Wires with clamps: Battery: Splints: Graduated cylinder: Sodium Carbonate: EPS/Biology Integrated Mrs. Dion and Mr. Hartshorn Name: _________________________________ EPS/Biology Integrated Lab Report Due Date: ___________________ Mrs. Dion and Mr. Hartshorn Electrolysis of Water: Semester 1 Formal Lab Report (Day 3) This is an outline of all of the sections for the lab. Each bullet represents something you need to include in your final lab report for each section listed below. Before you start Typing: DO NOT USE PERSONAL PRONOUNS (I, WE, MY, YOU, YOU). USE SPELL CHECK AND GRAMMAR CHECK TYPE ENTIRE LAB REPORT (EXCEPT DESIGN SET-UP) USE SCIENTIFIC VOCABULARY THIS IS FOR YOUR GRADUATION PORTFOLIO SO YOU NEED AN 80. DO YOUR BEST WORK. Introduction: Discuss the law of Conservation of Mass and how it is applied to balancing chemical equations. Define it using a quote from your book. Give an example of a balanced equation. Discuss the four main types of chemical reactions. Cite a source of information (it can be your text book: McLaughlin, C.W., Thompson, M., and Zike, D., 2005, Physical Science, McGraw-Hill Companies, Inc., Columbus, 894p. ) Problem Statement: “The purpose of this lab is to determine how the amount of Hydrogen gas compares to the amount of Oxygen gas produced in the electrolysis of water and to determine what type of chemical reaction will take place.” Discuss a few materials to be used in the lab and the methods used (Electrolysis of water). Hypothesis: Copy this information from your pre-lab (Day 1) Design Set-up: Add this from the lab day’s work (Day 2) Materials and Procedure: See lab day (Day 2)-add “refer to design set-up” as it applies to your procedure. This could increase your lab performance in this area to a 5. Data: Create the following data table: Electrode Gas Collection and Reactions Volume of Gas (mL) Reaction with Splint Positive Negative Give a reason why this data is needed to solve the problem statement. Describe the other visible information observed during the electrolysis. Name: _________________________________ Lab Report Due Date: ___________________ EPS/Biology Integrated Mrs. Dion and Mr. Hartshorn Results: Compare your results to those of the rest of the class. Graph the class data in a bar graph (Electrode on the x-axis, Volume of gas (mL) on y-axis) – Title the graph Discuss the trend in the volumes of the two gases below your graph. Conclusion–Critique of Hypothesis: Restate your hypothesis. State if it was accepted (correct) or rejected (wrong). Use facts (actual numbers) from the data to support your claim (hypothesis). Example: “The hypothesis was rejected because for every 1 part hydrogen that was collected, 4 parts of oxygen were collected.” Conclusion-Critical Connection: Identify possible sources of error (human and equipment). Discuss other problems that might be investigated using this experiment (e.g. Would other ionic compounds work as a catalyst?) How does this lab relate to real life? Give some examples. Works Cited: Provide at least one source of information (you may use your textbook or any other book or website) McLaughlin, C.W., Thompson, M., and Zike, D., 2005, Physical Science, McGraw-Hill Companies, Inc., Columbus, 894p. Title “Works Cited” page REMINDERS: DO NOT USE PERSONAL PRONOUNS (I, WE, MY, YOU, YOU). USE SPELL CHECK AND GRAMMAR CHECK TYPE ENTIRE LAB REPORT (EXCEPT DESIGN SET-UP) USE SCIENTIFIC VOCABULARY THIS IS FOR YOUR GRADUATION PORTFOLIO SO YOU NEED AN 80. DO YOUR BEST WORK.