Colony formation assay
advertisement

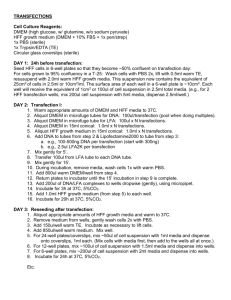
Colony formation assay OG 1-14-2001 These assays are based on the principle that certain proteins when expressed stably cause either cell cycle arrest or cell death, hence a reduction in colony number. 1. Plate out SAOS-2 (p53 -/-. pRB -/-), H1299 (p53 -/-. pRB +/+) or U2OS (p53 +/+, pRB +/+) in p60 dishes for transfection next day. The cells should be approximately 60-70 % (SAOS-2), or appr. 40-60 % (H1299, U2OS) confluent, respectively, on the day of transfection. Use 5 ml of medium per p60 dish. 2. Transfect the cells. You can either use BES calcium phosphate or Fugene-6 (Roche) – either one works well. Probably Fugene has lower toxicity, though. 3. With Fugene-6 transfect as follows: A. Aliquot the DNA’s (2.5 µg) into Falcon 2054 tubes. B. Prepare a master mix in a separate Falcon tube for all the transfections (make enough for one more transfection than you need because of pipetting errors). Mix 117.5 µl DMEM (without serum, or antibiotics) with 7.5 µl Fugene-6 per transfection. Incubate 5 min. C. Add the DMEM/ Fugene mixture (125 µl) to each tube with DNA and mix by piptetting up and down gently. Incubate 15 min at RT. D. Add the DMEM/ Fugene/ DNA mixture to the cells dropwise. E. The transfection mixture can remain on the cells overnight, since it’s not very toxic. Note on DNA to use: The DNA you transfect must have a drug resistance marker, preferably puromycin (it’s faster) but neomycin or hygromcyin may also be used. For this assay, pBabe-puro works quite well as a vector for puromycin selection. 4. The next day after transfection pass the cells from p60’s into p100 dishes. 5. Two days after transfection start the selection by adding the appropriate amount of selection drug. E.g. add puromycin to a final conc. of 1-1.5 µg/ml (for pBabe-puro). For G418 selection, 500 µg/ml is a good starting point. 6. Every 3-4 days the medium should be replaced with fresh medium containing the selection drug. Most of the non-resistant cells are dead after 3-4 days of puromycin selection. However, it takes 2-3 weeks for the drug-resistant cells to form a visible colony. 7. The assay should be stopped when the colonies are clearly visible even without looking under the microscope. Stain the colonies with crystal violet and count them if so desired. Notes: - Every assay should as a minimum have one positive and one negative control, i.e. the vector alone versus vector containing something ‘toxic’. - If you try to rescue colony formation with Bcl-2 or dn–p53 it can be done in an equal ratio, i.e. 2.5 µg ‘your construct’ + 2.5 µg Bcl-2/ dn p53 vector. Make sure whatever you try to rescue with does not have the same drug resistance marker. Also, when transfecting 5 µg, as in this case, double the amounts of everything (Fugene, DMEM ).