Focus formation assay in Rat
advertisement

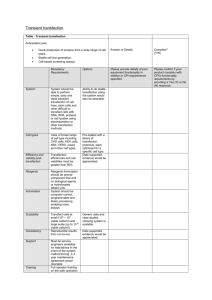
Focus formation assay in Rat-1 cells (N.B. according to Allan et al., Mol Cell Biol 2000 Feb;20(4):1291-8 the p21 promoter is methylated in Rat-1. Hence, although the p53 is wt, it fails to transciptionally upregulate p21) 1. Pass the Rat-1 cells a day in advance of the transfection (e.g. 1:5 from an 80 % confluent dish). Ideally, the cells should be 40-60% confluent when transfected. 2. Set up the transfection in the following way for a p100 dish (N.B. every assay should have at least a positive and a negative control, i.e. wt T (pos.) and vector or GFP (neg.)): - Add 10 µg SV40 large T expression vector with a selection marker into a Falcon 2054 tube. Don’t forget a vector control or GFP – the last one is good to double check your transfection efficiency- should be around 40 %. - Mix in a separate tube enough Fugene-6/DMEM for all the transfections + 1 to account for pipetting error. For every p100 transfection use 30 µl Fugene-6 + 470 µl DMEM (minus FCS, minus pen-strep). Incubate for 5 min at room temperature. - Add 500 µl of Fugene-6/DMEM to each tube with DNA, mix and incubate 15 min at room temperature. - Add the Fugene-6/DMEM/DNA mixture to the cells dropwise (should be about 10 ml complete medium on the p100 dish). Swirl to mix. - The Fugene-6/DMEM/DNA mix is relatively non-toxic and can stay on the cells 3. At 24 hrs post-transfection split the Rat-1 3:1:1 into three dishes 4. At 48 hrs after transfection start selection on the two dishes of sparser confluence. The type and concentration depends on which resistance marker you use. I usually use 1.0-1.5 µg/ml puromycin or 500 µg/ml G418. 5. The dish from 3. with most cells is the actual focus formation plate. Keep adding fresh medium every 3-4 days for 2-3 weeks until primary foci appear. Then stain these with crystal violet for visualization. 6. The two dishes that have been maintained under selection will probably be ready slightly sooner than the focus formation one. Take one of the selection plates and stain it with crystal violet. Colony numbers will reflect transfection efficiencies. The other selection plate should be trypsinized and pooled, after which large T expression can be confirmed in lysates from the pools.