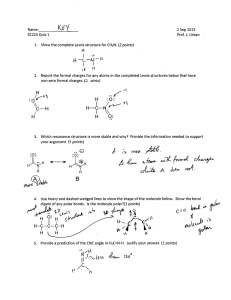
Name____________________ Period___________________ Chemistry Quiz
advertisement

Name____________________ Period___________________ Chemistry Quiz II.Give the VSEPR shapes that would result from the following situations. REPELLING PAIRS LONE PAIRS 3 1 4 0 5 0 2 2 3 0 2 1 2 0 6 0 I. SHAPE ANGLES _________________________ _________________________ _________________________ _________________________ _________________________ _________________________ _________________________ _________________________ Match the following phrases with the answers on the right. _____1 forms individual molecules _____2) due to electron transfer _____3) will generally dissolve in water _____4) share electron pairs _____5) poor to nonconductors _____6) made up of nonmetals _____7) usually solids at room temperature _____8) made up of metals and nonmetals _____9) representative unit is a formula unit _____10) liquids or gases at room temperature a) ionic b) covalent Below Indicate if the formula is ionic or covalent, name , draw the lewis dot structure, show the Polar bonds ( 0-Cl), Polar molecule yes or no, Shape of molecule. *indicates exceptions FORMULA I OR C NAME MgCl2 H2CO (C is central) NH4+ SeCl2 AsCl3 ClO2- CaCO3 CBr4 *SF4 omit DOT STRUCTURE POLAR BONDS POLAR MOLECULE SHAPE *KrF2