Chapter 5 - People.vcu.edu
advertisement
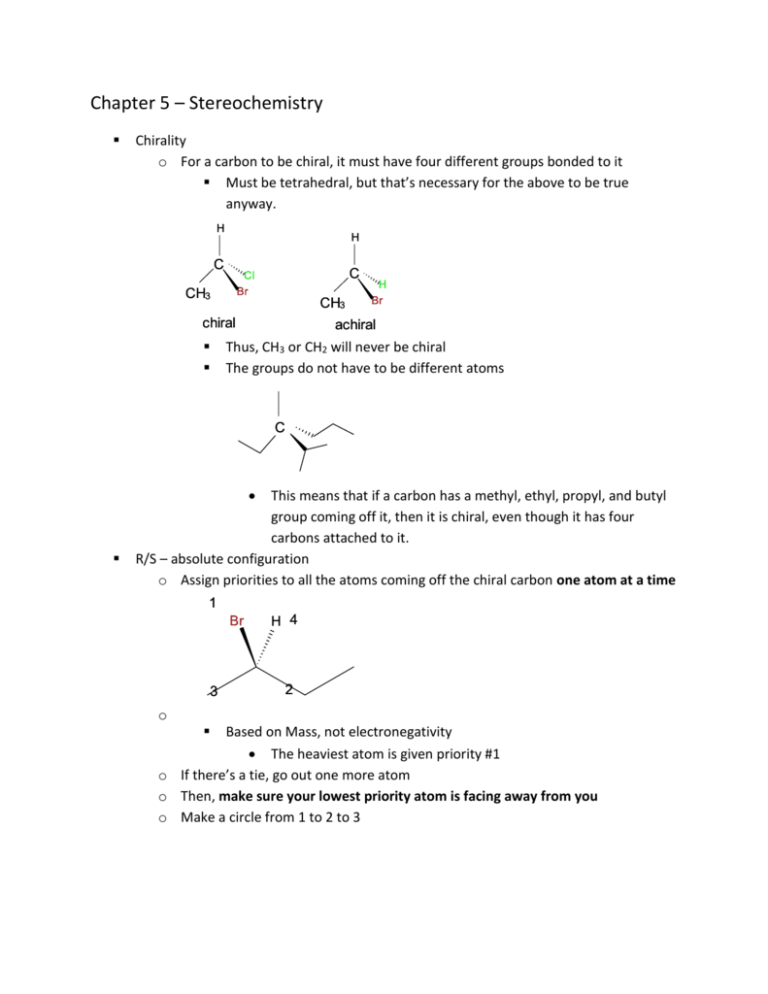
Chapter 5 – Stereochemistry Chirality o For a carbon to be chiral, it must have four different groups bonded to it Must be tetrahedral, but that’s necessary for the above to be true anyway. C C CH3 CH3 chiral achiral Thus, CH3 or CH2 will never be chiral The groups do not have to be different atoms C This means that if a carbon has a methyl, ethyl, propyl, and butyl group coming off it, then it is chiral, even though it has four carbons attached to it. R/S – absolute configuration o Assign priorities to all the atoms coming off the chiral carbon one atom at a time 1 4 3 2 o Based on Mass, not electronegativity The heaviest atom is given priority #1 o If there’s a tie, go out one more atom o Then, make sure your lowest priority atom is facing away from you o Make a circle from 1 to 2 to 3 1 4 2 3 o If the circle was clockwise, then assign R o If counterclockwise, then assign S What do I do if the #4 group is coming forward? o Still assign priorities to the different groups 2 3 1 4 o Find R/S as though #4 was going back. 2 3 1 4 o Switch it. This one looks like S, so we have an R. What do I do if the #4 group is in the plane? 4 3 2 1 o You have several options. o 1) Look at the molecule from another direction so that the #4 is facing away from you. This method seems to be easiest for those of you who like this chapter/ find this stuff easy. 4 3 look from over here 2 1 o 2) Rotate around a single bond to get the #4 group in the back. 2 4 4 3 2 3 1 1 o 3) Switch two groups, find R/S for the new molecule, and then switch it back. This one seems to be easiest for those of you who hate this chapter. 1 4 3 3 2 2 1 4 looks like an S, so it's an R Enantiomers vs. Diastereomers o An enantiomer is a non-superimposible mirror image All the R/S designations will be changed in enantiomers If there is a double bond, then its stereochemistry (cis/trans) will remain the same. o A diastereomer is a non-superimposible non-mirror image. Some but not all of the R/S designations will change. Cis/trans double bonds make your molecules diastereomers as well. How do enantiomers differ from one another? o Physical properties Most are identical Boiling point Melting point Density Optical activity is opposite Chiral compounds rotate plane-polarized light o If a compound rotates light clockwise, then it is called the + enantiomer. o Likewise, if it rotates light counterclockwise then it is called the – enantiomer. o Sometimes +/- is called d/l Older chemists use this, but you’ll see +/- in this course. Enantiomers rotate light the same amount, but in opposite directions. o So if one stereoisomer rotates light 4° to the right, then its enantiomer will rotate light 4° to the left. o It is important to understand that R/S configuration does not tell you whether you have the + or – enantiomer R/S configuration is a manmade convention, whereas +/- is a physical property. o It’s important to distinguish that R/S configuration has no relationship to +/-. 50/50 mixtures of enantiomers are called racemic mixtures or racemates. o These do not rotate plane-polarized light because the two enantiomers cancel each other out. Calculation of enantiomeric excess o We will not do this in this class. You are responsible for this in your lab lecture. o If you see a problem on the test which looks like it requires any sort of calculation with optical rotation, then you need to look at the question again. For example, sometimes you’ll see a question like “which of the following would rotate light 1° clockwise?” followed by a series of solutions. All but one of the answers will be achiral, so the answer is the only chiral answer. You might have an achiral molecule, an equal mixture of enantiomers, an equal mixture of diastereomers, and another achiral molecule. The correct answer would be the mixture of diastereomers because it is the only chiral solution given. o Chemical properties Chiral materials react with other chiral reactants selectively Think of the glove and hand model here If you have a bunch of right-handed gloves only right hands will fit. o Separation of enantiomers Very often tested! There are two ways to separate enantiomers 1) Convert to diastereomers, Separate, convert back to enantiomers 2) Chiral chromatography Mr. Baker wants to see all of the above if he asks this. If you skip “convert back” in number 1, you don’t get credit If you skip “chiral” in number 2, you don’t get credit Meso compounds o Compounds containing chiral carbons but which are not overall chiral This means a meso compound has not enantiomer. It will also not rotate plane-polarized light. o There must be a plane of symmetry present meso chiral Be sure you’re looking for a plane of symmetry and not an axis of rotation; the compound on the left has the plane of symmetry and the compound on the right has an axis of rotation. If no stereochemistry is shown, you cannot say whether it is meso or chiral. o Be careful not to be tricked by cyclic compounds with no chiral centers. These are achiral because there are no chiral carbons present. o Meso compounds do not have to be cyclic What is the relationship between these two compounds? They’re the same because if you rotate around the single bonds you can get them into symmetrical forms. They’re two different drawings of the same meso compound. Maximum number of stereoisomers possible for a compound o If n number of chiral carbons, then 2n possible stereoisomers Be sure to subtract any meso compounds from this total. How many possible stereoisomers are there of this compound? There are 2 chiral centers, so 2n=4. o But wait! o Here are the four versions given by this formula o The top two of these structures are meso, so we have to subtract one from the four. There are three total stereoisomers of 1,2dibromocyclohexane. o Common question for this material How many monochorinated products, including stereoisomers, will you get when you chlorinate a particular compound. Ex. First, find all the positions that give you distinct structural isomers. Then, go through and see if adding a chlorine at each spot makes any spot chiral. If it doesn’t, then you only get one version of that chlorination. If it does, you get 2n products where n=the number of chiral carbons. Back to our example, here’s how I would do it. If I put a chlorine here, nobody becomes chiral. That said, you can still get the chlorine placed either cis or trans to the alkyl group that’s on the ring, so you get two versions. o I almost always miss this cis/trans possibility so make sure you don’t make my mistake! If I put a chlorine here, two positions are now chiral so there are four versions of this chlorination. * * The same thing happens here. * * No new chirality, so just one version here. Here, you get one chiral center so there are two versions * Here you have no chirality. Here you get four products again. * * And at the last position you get one chiral center so 2 products. * If you kept track of this as you did this, then you would have something that looks like this: 1,2 3,4,5,6 7,8,9,10 11 12, 13 14 15,16,17,18 19,20 o If you see, I just kept a tally of how many isomers we had gotten to at each spot. o So here, there are 20 total monochlorinated products possible. Allenes o Chiral compounds with no chiral carbons o Two double bonds directly next to each other A C B D o For the molecule to be chiral, A≠B AND C≠D Fischer Projections CO2H H2 N H CH3 o Every intersection is a chiral carbon. o Everything on the horizontal is coming forward and everything on the vertical is going back CO2H H2N H CH3 If you are asked to convert from Fischer to line-angle you cannot just draw the dashes and wedges like I’ve done here. Two of the pieces coming off any atom must be drawn flat (in the plane). o Still assign priorities the same way as before 2 CO2H H2 N 1 H 4 3 CH3 o Draw your circle from 1 to 2 to 3 2 CO2H H2 N 1 H 4 3 CH3 o If H (or any lowest priority group) is on the Horizontal, it’s Horribly Wrong! It looked like an R, but H was on the Horizontal so it’s an S. Comparing two molecules drawn as line-angle and Fischer What is the relationship between these two molecules? CH3 H Br HO H HO CH2CH2CH3 o Method #1 – Assign R/S This is the easiest way for most people. CH3 S H Br S HO R H HO R CH2CH2CH3 Both configurations are reversed, so these are enantiomers. o Method #2 – Advanced method which is quicker but harder for most Remember that with a Fischer you are looking at the molecule so that the vertical pieces are going back and the horizontal pieces are coming forward. Stay with me here. Because of the implied stereochemistry of a Fischer, you are basically looking at the “peaks” of the chain. eye HO eye Using this, you can easily convert to a Fischer from the line-angle and then compare the two Fischers. When you look down at the carbon with the bromine, you see that you have a CH3 on top, an H on the right, a Br on the left, and the rest of the molecule going down. CH3 Br H rest o View of this carbon from above. When you look up at the carbon with the OH, you have the OH on the right and an H on the left. CHBrCH3 OH H rest o View of this carbon from below. Special stereochemistry designations for biological molecules Sugars and amino acids are chiral When you draw each in Fischer form, they have special stereochemical designations. Amino acids I If the amino group is on the left, it is an L-amino acid. CO2 H H2 N H CH3 L-alanine o This is the natural form. If the amino group is on the right, it is a D-amino acid. CO2 H H NH2 CH3 D-alanine Sugars If the last chiral carbon has the OH on the left, then it is an Lsugar. CHO HO H H OH HO H HO H CH2 OH L-glucose If the last chiral carbon has the OH on the right, then it is a Dsugar. CHO H HO OH H H OH H OH CH2 OH D-glucose To switch between the L and D form, you switch all the chiral carbons, not just the bottom one. CHO H HO H HO OH H OH H CH2 OH not glucose (but definitely L)