Catatan_Kuliah II
advertisement

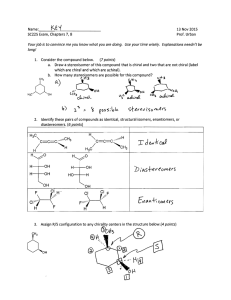
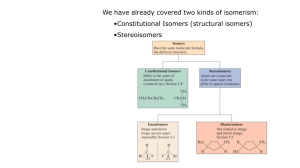
Stereoisomer Descriptor Stereoisomer Enantiomer Diastereomer Erythro, threo Meso Compound Epimer Chiral Molecules With No Chiral Centers Definitions Stereoisomers – compounds with the same connectivity, different arrangement in space Enantiomers – stereoisomers that are nonsuperimposible mirror images; only properties that differ are direction (+ or -) of optical rotation Diastereomers – stereoisomers that are not mirror images; different compounds with different physical properties enantiomer Erythro dan threo Diastereomer: Stereoisomer yang bukan pasangan enantiomer 1 dan 2 enantiomer 3 dan 4 juga enantiomer Diastereomer: 1-3; 1-4; 2-3 dan 2-4 Epimers: distereomer yang berbeda hanya pada satu pusat kiral saja OH OH HO OH HO OH HO OH HO OH OH OH Senyawa meso Chiral Molecules With No Chiral Centers Allenes can be Chiral H Cl H C C H C C CH3 CH3 C C H Cl Mycomycin, an antibiotic H H H C C C C C C C CH=CHCH=CHCH2CO2H o Nocardia acidophilus []D = -130 PPh2 PPh2 (S)-BINAP PPh2 PPh2 (R)-BINAP Specifying Configuration (Chirality); Cahn-Ingold-Prelog Notation Specifies the "configuration" at each chiral center, equivalent to "cis-" and "trans-" etc. Abbreviated rules Assign priorities to groups attached to chiral center according to atomic # (same as Z/E rules) Compare 2nd, 3rd etc. atom from center as necessary, look for first point of difference Multiple bonds "add up" Determine direction of rotation 1 > 2 > 3 looking with #4 group pointing "away" Prioritize Using Cahn-Ingold-Prelog Rules Look at atoms directly attached to chirality center a) higher atomic number = higher priority b) heavier isotopes = higher priority If no difference at first attached atom, move along the chain until the first point of difference. Multiple-bonded atoms are equivalent to the same number of single-bonded atoms.