Scientific abstract
advertisement

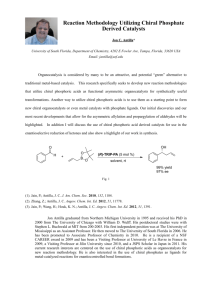
Abstract For a long time chiral silicates remained elusive due to the Berry pseudorotation. In order to synthesize these silicates, the corresponding silanes have to be produced. Recently, a new synthetic route for the asymmetrical synthesis of silanes was reported by Kuninobu et al., where a rhodium catalyst with a chiral ligand was used to obtain higher ee values. The importance for obtaining higher ee values is because these pentaorganosilicates possess chirality, making them prone to racemization because of the Berry pseudorotation. Previous reports show that the Berry pseudorotation can be inhibited through steric hindrance and by introducing a strong electronegative monodentate group, resulting in configurationally stable chiral silicates. The synthesis for racemic spirosilanes already existed, but the synthesis is less favorable than the one adapted by Kuninobu et al. because of asymmetric purposes. The first aim of the research was to obtain three spirosilanes, all with different substituents, and to observe if they are adequate to inhibit the berry pseudorotation. Unfortunately, there was not enough time to synthesize two of these spirosilanes. However, the spirosilane with the substituent 2-phenylbenzo[b]thiophene was successfully synthesized. A second aim of this research was to investigate if it was possible to get a better separation of the successfully synthesized spirosilane, by varying the concentration of the rhodium catalyst and chiral ligand (R)-(-)-DTBM SEGPHOS. At the same time the ee value would be determined for various concentrations and the best concentrations for a better separation would be determined. Furthermore, a second chiral ligand (R)-(+)-SEGPHOS was used in a rhodium catalyzed reaction with the concentration which had the best separation, in this case being the highest concentration of rhodium catalyst and chiral ligand. For the reaction with the chiral ligand (R)(+)-SEGPHOS a lower %ee of 27 was observed compared to the chiral ligand (R)-(-)-DTBM SEGPHOS which had an %ee of 55. However, a significantly higher isolated yield could be obtained using (R)-(+)-SEGPHOS.