Name
advertisement

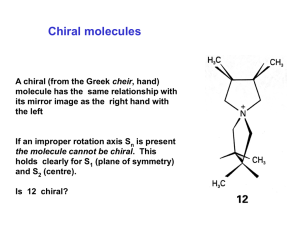
Name____________ Organic Chemistry I Final Exam 200 Points December 2014 1) Draw the electron configuration diagram for the following elements. [I don’t want a nucleus with electron orbitals drawn around it.] (9 points) a) Lithium c) Fluorine b) Neon 2) What is the hybridization of the molecular orbitals on the following molecules? (6 points) a) Carbon involved in only single bonds b) Carbon involved in a double bond c) Carbon involved in a triple bond 1 3) Name (IUPAC rules) the following structures. (8 points) 4) Professor J. P. Sullivan took his new compound he synthesized and found it has a specific rotation of ([])= +16o for the R enantiomer. After placing his sample in the polarimeter he had an observed rotation of +4o. a) What enantiomer is in excess? (3 points) b) What is the enantiomeric excess of this enantiomer? (3 points) c) What is the % of R and % of S in the mixture? (3 points) 2 5) Draw two diastereomers of the following Compound. (6 points) H CH3 Br CH3 Br H 6) What type of reaction would occur under the following conditions? (9 points) YOUR CHOICES ARE [SN2, E2 or SN1/E1 mix] a) You’re reacting a tertiary haloalkane with a really weak nucleophile. b) You’re reacting a secondary haloalkane with strongly basic hindered nucleophile. c) You are reacting primary haloalkane with a weakly basic unhindered nucleophile. 7) Draw the mechanism and the product for the following reaction. . (Make sure your arrows are pointed exactly where they should be and the you include all lone pairs of electrons) (5 points) 8) Draw the Newman projections for lowest and highest energy formations of butane and ethane (8 points) 3 9) Draw the resonance form(s) and the resonance hybrid for the following structure. (6 points) O CH3 O 10) Fill-in the blank/ short answer (22 points) a) Using this reagent on primary alcohols results in a carboxylic acid product. b) A _____________ functional group shows up around 200 PPM on a carbon spectrum. c) LDA is a base that is primarily used for a(n) ________ reaction. d) The least stable Newman projection of ethane is called the _________ formation. e) A carbocation forms when a carbon has only ___________ bonds. f) A reaction in which the molecule reacts with itself is called an ______________ reaction. g) The most stable chair form results when the largest substituent is in the __________ position. h) A _________________ reaction results in a C-C bond formation along with reducing the molecule and making an alcohol. i) An alcohol will behave like a(n) ______________ when it is in a very acidic environment. j) A stereoisomer that has 2 or more chiral centers and not related as a mirror image is called a __________. k) When –SH is a substituent (not part of parent) it is called a ______________ group. 4 11) Fill-in the following products (major product only) or reagents: (30 points) OH 1) 2) H+, H2O O OH PBr3 Br LDA O 1) LiAlH4 2) H+, H2O HO O 5 12) a) Name one diastereomer of (2R, 3S) 2,3dibromobutane. (3 points) b) Name the enantiomer of (2R, 3S) 2,3dibromobutane. (3 points) 13) Multiple Choice (20 points) a) The most stable carbocation is a ______________. i) primary carbocation ii) secondary carbocation iii) tertiary carbocation iv) complexed with sodium b) The best reagent to undo a sodium borohydride reaction with a ketone is _________. i) a Grignard reaction ii) doing an E2 reaction iii) a chromium reagent iv) LiAlH4 c) The best reaction to create R-2-Iodobutane from S-2-bromobutane is _______ reaction i) an alkylithium ii) an acid hydrolysis iii) a Sn2 reaction iv) an E2 reaction 6 d) In a Fisher projection, the horizontal axis denotes bonds that are __________. i) chiral ii) facing backward iii) facing forward iv) in the plane e) The amount of one enantiomer over the other in a mixture is denoted as _________. i) EA ii) RS iii) EE iv) ES 14) Define as R or S every chiral center in the molecules below. (12 points) H CH3 HO CH3 H Br H CH3 H H OH H OH OH CH3 CH2CH3 HO H Br H Cl Cl Cl Cl 7 15) What is the formal charge on the indicated atoms? [I checked them and they are how I want them. In other words, don’t change the structure](6 points) O CH3CH2CHCH2CH3 N H H 16) Draw (in the chair form) the most stable conformation of cis -1 methyl 3- butoxy cyclohexane (5 points) 17) Devise a synthesis of 3-pentanol from propionaldehyde and any other reagents you wish. (5 points) [You do not have to show the mechanism] O propionaldehyde 8 18) For the following molecules: a) Indicate with an * any chiral centers (4 points) b) Identify the molecules as chiral, not chiral or meso (4 points) Br Br H H H H H H H OCH3 H Br Br H H H 19) From the given spectra and data. Identify the compound by drawing its structure. (20 points) [Make sure you write down all your work to get partial credit and you circle your final structure] a) C5H10O 1H-NMR: (PPM) 1.23 (doublet, 6H); 2.75 (Septet, 1H); 3.2 (singlet, 3 H) 13C-NMR: (PPM) 22, 26, 42, 203 9 b) 10