is mycobacterium avium subsp.paratuberculosis an etiological
advertisement

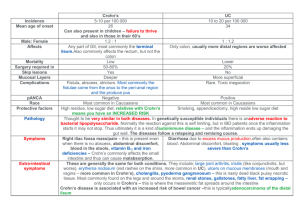
ISRAEL JOURNAL OF VETERINARY MEDICINE Vol. 59 (4) 2003 IS MYCOBACTERIUM AVIUM SUBSP.PARATUBERCULOSIS AN ETIOLOGICAL FACTOR IN CROHN'S DISEASE? Rudoler N. Visit of Infectious Diseases, Soroka Medical Center, Beer-Sheva, Israel Abstract Paratuberculosis is a chronic inflammation of the intestine in animals caused by the slow growing and fastidious bacterium, Mycobacterium avium subsp.paratuberculosis (MAP). The main clinical features of the disease include chronic diarrhea, a drop in milk yield, and progressive, afebrile, weight loss that leads to emaciation. Crohn's disease is a chronic inflammation of the intestine in humans whose etiology is unknown. Immunological factors, infectious agents, genetic and environmental factors are proposed as possible causes. Crohn's disease is characterized by chronic diarrhea, abdominal pain, fever, anorexia and weight loss. The pathological and clinical similarities of the two diseases have raised the hypothesis that MAP may be involved in the pathogenesis or is a direct cause of Crohn's disease. In this literature review we summarize the published data on the possible connection between MAP and Crohn's disease. The review is sub-divided according to clinical and laboratory findings. The clinical evidence is based on trials in which antimycobacterial therapy was prescribed for Crohn's disease patients. The laboratory evidence consists of several methods (primarily PCR) used to identify MAP from Crohn's disease patients. The accumulated data shows that there is no clear-cut relationship between MAP and Crohn's disease. Prospective studies are needed to test this hypothesis over a wider range of possible etiologies that include combination of infectious, immunological and genetic factors. Introduction Paratuberculosis (Johne’s disease) is a chronic digestive tract disorder of both wild and domestic ruminants. The disease is characterized by granulomatous enteritis that induces a progressive weight loss and terminates in the animals' death. Paratuberculosis is both a contagious and enzootic disease of ruminants caused by the multiplication of a specific bacterium, Mycobacterium avium subsp paratuberculosis (MAP) in the intestinal mucous membrane. The infection can be transmitted by either direct or indirect contact of infected animals with susceptible animals, and occurs mainly by the fecal - oral route. Bacilli are ingested in large numbers most commonly when young animals nurse on teats contaminated by feces of infected animals. Infection is also caused by ingestion of contaminated feed and water, the organism can also be shed in the colostrum and milk of infected cows, and intrauterine infections have also been described. Infection is acquired early in life, but clinical signs rarely develop in cattle less than 2 years old. There appears to be an age-related resistance to the development of new paratuberculosis infections. Animals infected in the first months of life are the most susceptible, while adult animals are quite resistant. Whether or not the exposed calf becomes infected depends on the number of bacilli ingested and the host defense mechanisms (1,2,3). MAP is an obligate intracellular pathogen; it lacks the iron-chelating compound mycobactin, and thus can only survive in a host that can provide the iron required for growth (4). It is closely related to other M.avium bacteria, sharing some antigenic determinants(1). MAP is differentiated from the other mycobacterial species which are common in the environment by its ability to cause bowel disease in cattle and other animal species (3). The main distinguishing feature of MAP is its slow growth and the dependency on exogenous mycobactin for in vitro growth (1). Crohn’s disease is a chronic inflammatory bowel disease of which the cause or causes are not yet established. Chronic diarrhea with abdominal pain, fever, anorexia, weight loss and a right lower quadrant mass are the most common presenting features of Crohn’s disease. Although primarily considered a disease of the small intestine it can affect any part of the gastrointestinal tract and sometimes extraintestinal sites (the most commonly affected site is the distal ileum and the colon). Current concepts regarding the cause of Crohn’s disease emphasize a dysfunction of the immune system resulting in a prolonged and intense process of inflammation. The damage to the bowel appears to be due to this inflammatory process (3). The pathogenesis of Crohn’s disease probably involves an interaction of genetic and environmental factors, but the precise mechanism responsible for initiating chronic intestinal inflammation remains unclear. Three theories of disease mechanisms in Crohn’s disease are currently under consideration: (i) reaction to a persistent intestinal infection, (ii) existence of a defective mucosal barrier to luminal antigens, (iii) a dysfunctional host immune response to ubiquitous antigens, cytokine imbalance and the breakdown of tolerance to gut microbial flora. Cigarette smoking has also been linked to the long term course of Crohn’s disease. It is possible that an infectious agent is also involved in the etiology of Crohn’s disease, and of those incriminated as possible causes, Campylobacter jejuni, Campylobacter faecalis , Listeria monocytogenes,Escherichia coli, Mycobacteria spp are prominent. In 1913, Dalziel (4) reported several cases of chronic intestinal enteritis in humans that were similar to intestinal tuberculosis but did not feature acid-fast bacilli. He speculated that these human cases and Johne’s disease (=paratuberculosis in ruminants) might have the same etiology. In 1932, what is now known as Crohn’s disease was distinguished from intestinal tuberculosis, but suspicion remained that it might have a mycobacterial origin. Crohn’s disease in humans and Johne’s disease have similar clinical and pathological features. Both are characterized by chronic diarrhea and weight loss and by granulomatous appearance of the diseased tissue. Interest has remained high since 1984 when Chiodini repeated Ward Van Patter's findings from the 1950’s that employed techniques to isolate MAP from animals (4). Chiodini reported the isolation of uncharacterized mycobacteria from tissues of three patients with Crohn’s disease (4). The main way that humans become infected is by ingestion of infected water or milk. Several reports have emphasized the crucial role of MAP surviving high-temperature, short time pasteurization if they are present in raw milk in sufficient numbers (more than 10 cells/ml) (5,6,7,8). In addition, the epidemiology of the disease, whose incidence is rising in western societies, concurrent with the low rates of Crohn’s disease in developing nations over the second half of the 20th century and high rates among immigrants to western societies, is consistent with the possibility that a critical infection may be acquired from cattle or sheep via milk or meat ingestion, and the emergence of Crohn’s disease in patients with the appropriate genetic predisposition (9). The aim of the article The objective of the review is to examine the evidence that Crohn’s disease is associated with MAP infection. The importance of this issue to public health is clear: it is a pathogen with a prevalence rate of infection ranging between 21 and 70% in herds of Western Europe and North America (10). It can survive commercial pasteurisation conditions in routine use (subclinically infected cows may secrete MAP in milk). It is found in the potable water supply of large cities in industrialized countries and also survives higher concentrations of chlorine (two parts per million) than the 1.1 parts per million routinely present in first-use municipal water in the USA (10,11). These issues are of extreme epidemiological and clinical importance since specific anti-mycobacterial therapy may improve the quality of life of Crohn’s disease patients. Methods The review was based on a PubMed search of publications between 1966 and 2004. Only articles that provided sufficient clinical or laboratory information on the association between MAP and Crohn’s disease were included. The following key words were used: Mycobacterium paratuberculosis; Crohn’s disease; etiology; therapy. Results The review will discuss the laboratory and clinical evidence. The laboratory evidence includes 3 articles dealing with diagnosis by culture, 11 studies employing PCR as the method of diagnosis, 2 articles on serological methods and 15 articles that present clinical evidence of a link between MAP and Crohn’s disease. Laboratory evidence Cultural detection of MAP in Crohn’s disease Three articles dealt with a possible connection between MAP and Crohn’s disease and are based on culturing of the organism. In these articles the culture times were very long (the shortest took 12 weeks (9),the median was between 14 and 88 weeks (13), and the longest yielded acid fast bacilli or spheroplasts after between 2 and 6 years (12)). In the first study, seven breast milk samples comprising 2 Crohn’s disease patients and 5 control mothers were cultured. All five control samples were negative for MAP in all cultures, while MAP was isolated from the cultures of the Crohn’s disease patients which were were also confirmed by hybridization (9). In the second study (12), seven of 31 cultures were MAP-positive, and six of these were from Crohn’s disease patients. Another positive culture of MAP was detected in a sample taken from a non-inflammatory bowel disease patient. This positive culture also grew acid-fast bacilli which were not MAP. The third article that MAP was detected in 14 of 33 (42%) Crohn’s disease patients, and 3 of 33 (9%) of non-inflammatory bowel disease controls (13). Detection of MAP in Crohn’s disease by PCR The extremely long incubation periods and the very close similarity with M.avium, (a species ubiquitous in the environment, and also in the human gut), has stimulated the use of DNA probe technologies. The discovery of IS900 , a species-specific, repeating DNA insertion element in the MAP genome was an important step forward. The use of the polymerase chain reaction (PCR) to amplify fragments of IS900 permits rapid and accurate detection of MAP DNA from tissue without the need for laborious and time consuming culture (14,15).The review dealing with detection of MAP by PCR and culture includes 11 publications . We have summarized them according to whether they support or disclaim the involvement of MAP in Crohn’s disease. Four of the 11 studies do not support the hypothesis that MAP is involved in Crohn’s disease. In the study of Rowbotham and others, samples of intestinal tissue were obtained from patients undergoing either colonoscopy or surgical resection. These included 68 patients with Crohn’s disease,49 patients with ulcerative colitis, and 26 non- inflammatory bowel disease controls. In no case was MAP detected in any of the inflammatory bowel disease tissue samples while only one non-inflammatory bowel disease case was positive(14). In Suenaga and others' study, three biopsy specimens were obtained from the rectosigmoidal colonic mucosa using colonoscopy in 10 patients with Crohn’s disease and 18 patients with ulcerative colitis. Sixteen noninflammatorybowel disease controls were also examined. IS900 sequences were detected in all of 10 patients with Crohn’s disease, in 11 of 18 patients with ulcerative colitis, and in 14 of 16 control patients with non-inflammatory bowel disease (16). In another article, mycobacteria were found in 17 out of 36 (47%) patients with Crohn’s disease, 6 out of 13 (46%) in patients with ulcerative colitis, and 13 out of 23(57%) control tissue samples. No MAP was detected in any of the samples (17). In addition, other results which failed to support the involvement of MAP stress in 25 specimens tested from 21 Crohn’s patients: only 1 positive specimen was noted, whereas the 8 specimens from the 5 ulcerative colitis patients and 11 samples from control patients were negative by polymerase chain reaction. None of the cultures grew MAP (18). Five reports support the hypothesis that MAP is involved in Crohn’s disease. In the first study, PCR was applied to DNA extracts of full thickness samples of intestine removed at surgery from 40 patients with Crohn’s disease, 23 patients with ulcerative colitis, and 40 control patients without inflammatory bowel disease. MAP was identified in 26 of 40 (65%) Crohn’s disease, in 1 of 23 (4.3%) ulcerative colitis, and in 5 of 40 (12.5%) control tissues (19). PCR was also done on the positivecultured milk samples, and provided 99% homology with the IS 900 sequence (9). Dellísola and others used the IS 900 sequences of MAP and found them in 13 of 18 samples (72%) from patients with Crohn’s disease, in 1 of 5 with ulcerative colitis, in 2 of 6 with severe unclassified colitis (20). Nine of 36 Crohn’s disease patients were found positive by PCR in comparison with 3 of 43 patients with ulcerative colitis and noninflammatory bowel disease according to the study of Hulten and others (21). The most convincing evidence concerning the link between MAP and Crohn’s disease that was challenged by the PCR, is demonstrated by Bull and others (13). In this study, MAP was detected in inflamed ileal and colonic mucosae of Crohn’s disease patients in 92% of cases, regardless of whether granulomata were found microscopically. The last three studies employing PCR and culture as the method of choice for detecting MAP did not reach a clear- cut conclusion concerning the involvement of MAP in Crohn’s disease. Four of 31 Crohn’s disease tissues and none of 30 control and the ulcerative colitisderived tissues amplified MAP (15). The second study indicated that 6 of 18 of Crohn’s disease cultures and one of six of non-inflammatory bowel disease control were MAP- positive. The intensity of the IS 900 PCR signals indicated very low numbers of MAP organisms and bore no relation to visible spheroplastic or bacillary mycobacterial growth (12). The third study failed to identifiy M.avium in tissue samples taken from Crohn’s disease patients but were identified in the control group, however (22). Several possible explanations for the discrepancies in PCR experiments have been raised. The first is geographical variability in MAP infections. It is conceivable that Japan, an area with a large number of PCR-positive Crohn’s disease patients, has a high prevalence of MAP enteritis. Another explanation is differences in the PCR technique. A third reason for the discrepancy in PCR experiments is sampling error. In addition, in situ sampling techniques, specifically the presence of granulomata, have been implicated as a variable in the detection of MAP by PCR (18). Granulomatous Crohn’s disease was significantly more likely to contain MAP than non-Crohn’s disease tissue controls. There are a number of reasons why Crohn’s disease tissues with granulomata can contain more MAP DNA. The most obvious is that that this organism causes Crohn’s disease in a subgroup ( 20%) of patients with this histopathological appearance. Another possibility is that granulomatous tissue is more likely to develop infection with this organism but is not its cause (15). Two articles (13,21)report that IS900 sequences were detected in 65% Crohn’s tissues and in 25% of children without inflammatory bowel disease. A low prevalence rate of IS 900 DNA was detected in tissues from controls suggesting an unexpected prevalence of this pathogen in the alimentary tract of normal healthy humans. These studies also suggested that MAP could be spread from infected cattle to humans through ingestion of milk or water (16). Serological detection of MAP in Crohn’s disease MAP is very closely related to other MAC (Mycobacterium avium complex) and organisms of M. intracellulare complex. MAC's are widely distributed in the environment and can be isolated by fecal culture from healthy persons. A serological study in 1980 attempted to identify agglutination of three strains of MAP by means of Crohn’s disease sera. No response was seen with two of the strains, while the agglutination observed with the third MAP strain showed no difference between Crohn’s disease and normal sera(1). Stainsby and others examined whether a specific serum antibody response to mycobacteria occurs in Crohn’s disease or ulcerative colitis. Sera from 38 patients with Crohn’s disease, 15 with ulcerative colitis, and 30 healthy control population were assayed in an enzyme immune linked immunosorbent assay (ELISA). In addition, IgG, IgM, and IgA levels to MAP were determined in sera from patients with active and inactive Crohn’s disease and the control population. There was strong evidence of contact with enviromental mycobacteria in all patients and control population , with the greatest responses to M.avium, M.tuberculosis, and M.kansasii. There were no differences in antibody levels to MAP in patient and control groups . Although a subset of patients with active Crohn’s disease (25%) had IgG concentrations that exceeded the control mean by more than 2 SD, this finding may not be specific to Crohn’s disease (24). In another study of the seropositivity rate among Crohn’s disease patients, ulcerative colitis, healthy controls and unaffected siblings, the researchers found no differences between the groups( 37.8%, 34.7%,33.6%,34.1% ) of the Crohn’s disease patients, ulcerative colitis, healthy controls and the unaffected siblings,respectively (23).Both sources agree that serological investigations cannot provide consistent and convincing evidence of raised serum antibody levels to mycobacteria in Crohn’s disease. The raised levels reported in some studies may represent sensitivity as a result of exposure to ubiquitous environmental strains of mycobacteria. It is well documented that patients with Crohn’s disease often have an increased antibody response to other microorganisms , including enterobacterial species and Saccharomyces cerevisiae (23). As such contact with these organisms and environmental mycobacteria is via the oral route, presumably through a defective barrier function of the gut mucosa, it is proposed that the organisms may be just “passing through”. Given the taxonomic similarities between mycobacterial species, it is not surprising that there is extensive cross-reactivity between MAP and both M. kansasii and M.tuberculosis. In addition, it has been suggested that the lack of a distinct immune response to certain mycobacterial antigens may be the result of a lack of these antigens in the cell wall-deficient forms thought to cause Crohn’s disease (24). Clinical evidence Treatment of Crohn’s disease by anti-mycobacterial drug We found 15 articles that dealt with the effects of anti-MAP therapy in the clinical course of patients with Crohn’s disease. Of these, 7 were controlled trials ,3 were prospective open label trials, 2 were case reports and 3 studies were unclassified (Table 1). Clinical improvement as a result of successful treatment, may be the best evidence for the existence of some link between MAP and Crohn’s disease. The main parameter used to asses the clinical condition in the above studies was a Crohn’s disease activity index. This index was improved in 7 of 10 trials that used it.Only 3 trials (29,34,38) reported lack of improvement or the absence of statistically significant results between the control and active treatment groups. The main criterion is variable length remission, and complete resolution was achieved only in one case (36). Complete anatomical healing is difficult to achieve because this needs specific drug combinations that are not always well tolerated by the patients, the length of the treatment, and the patient's characteristics. A longer period of treatment is generally necessary for cure of some mycobacterial diseases, while multiple drug resistance commonly develops during treatment. Also Johne’s disease of cattle is incurable to date (30). Another possible explanation for anti- MAP therapy to induce remission could be due to the possibility that Crohn’s disease has several causes involving an interaction between infectious or environmental triggers, the immune response and genetic susceptibility. MAP may cause Crohn’s disease in only a proportion of patients while some unknown etiology is responsible for initiating disease in other patients (25). In contrary to the above successful results some claim that drug-induced clinical improvement of Crohn’s disease should be interpreted with caution for several reasons. First, antibiotics have been shown to be effective in controlling the activity of Crohn’s disease. Second, as the course of the disease is variable and “spontaneous” decrease of disease activity may be seen in 26-42% of patients (31). Discussion In this review, we have tried to evaluate whether MAP is the etiologic factor involved somehow in the pathogenesis of Crohn’s disease or acts only as a microbial bystander . Those who support the view that MAP may be involved or cause the disease claim that when treating Crohn’s disease patients with specific anti-mycobacterial drugs, either clinical remission (25,28,,30,36,37,), a decrease in Crohn’s disease activity index (26, 27, 28, 30, 33, 39, 41) or complete resolution (37) can be achieved. In addition, in Crohn’s disease patients, MAP DNA has been detected by PCR in up to 90% of cases, by in-situ hybridization in 70% of cases, and by RT-PCR for MAP RNA in 100% of cases (21,42). MAP has also been cultured from human milk (12), feces, intestinal tissues, and peripheral blood of patients with Crohn’s disease (43). A recent development is attributed to the finding of NOD2, a family of cytosolic proteins implicated in intracellular recognition of bacterial components. This finding indicates that the presence of certain bacteria in the face of permissive NOD2 variants (as they occur in some Crohn’s disease patients) permits the establishment of a persistent mycobacterial infection and the development of Crohn’s disease (43). In contrary to these arguments, there are some other propositions dealing with the role of MAP in Crohn’s disease. First, there are competing etiologies for Crohn’s disease, such as genetic predisposition, enviromental factors within the gut related to dietary factors or the microbiological enviroment and an abnormal immune reaction. For the infectious disease hypothesis to be accepted for any organism, Koch’s postulates need to be fulfilled. To date, there is no evidence that high risk groups susceptible to the disease (e.g. families of cattle and sheep farmers, abattoir workers, etc) exist with a higher incidence of Crohn’s disease. Another claim is that MAP has been isolated from individual cases of Crohn’s disease whereas it has been grown from individuals without the disease (10,16). Other arguments include failure to detect MAP markers in Crohn’s patients when compared to ulcerative colitis patients (10,14 ,16,17) and failure of clinical improvement in patients treated with anti-mycobaterial drugs (14, 31, 34, 35, 38). There are pathological similarities between Johne’s disease and Crohn’s disease but there are also many features that are absent (1) . Van Kruiningen (10) suggests that the closest human disease to Johne’s disease is the Mycobacterium avium intracellular infections of AIDS patients, rather than Crohn’s disease. According to the above sources (5,6,7,8,9,11,23) the potential exists of MAP as an emerging zoonosis which is transmitted through milk, undercooked beef and water. Three articles (3,11, 42) that stress the steps taken in the UK to minimize the entry of MAP into the food chain and the policy that the USA should consider this threat have been published. At present, most agree that Crohn’s disease is best considered as a syndrome with different etiologic factors with similarities in presentation (1,43). The organism is relatively common and it is likely that many people have come into contact with it. The organism may have a role either as a causal agent , a secondary invader which exacerbates the disease, or as a non-pathogenic colonizer because of changed bowel conditions (1). Up to date, the possible link between MAP and Crohn’s disease as an etiological factor of the disease has not been confirmed. There is need of better prospective studies to elucidate this connection. According to these findings, it is impossible to recommend any specific anti-MAP therapy to Crohn’s disease patients. LINKS TO OTHER ARTICLES IN THIS ISSUE References 1. Possible links between Crohn’s disease and paratuberculosis. Report of the scientific committee on animal health and animal welfare, Europe. eu.int/ comm/ food/ fs sc/ scah/ out_38 pdf. 2. Johne’s disease (paratuberculosis); The Merck Veterinary Manual, eighth edition, Merial, p.537-539, 1998. 3. Mycobacterium paratuberculosis: Does it contribute to Crohn’s disease? Food Safety Authority of Ireland, www.fsai.ie/publications/other/myco.pdf. 4. El-Zaatari F.A.K, Osato S.M and Graham D.Y. Etiology of Crohn’s disease: the role of Mycobacterium avium paratuberculosis. Trends in molecular medicine, 2001. 5. Grant I.R, Hitchings E.J, McCarthney A. Effect of commercial -scale hightemperature, short-time pasteurisation on the viability of M.Paratuberculosis in naturally affected cow’s milk. Applied and environmental microbiology, 2002; 68:602-607. 6. Miller D., Ford J., Senderson J. IS900 PCR to detect M.Paratuberculosis in retail supplies of whole pasteurized cows’ milk in England and Wales. Applied and environmental microbiology, 1996; 62:3446-3452. 7. Sung N. and.Collins M.T., Thermal tolerance of M. Paratuberculosis. Applied and environmental microbiology, 1998; 64:999-1005. 8. Grant I.R, Ball H.J, Neil S.D and Rowe M.T. Inactivation of M.paratuberculosis in cows' milk at pasteurization temperatures. Applied and enviromental microbiology, 1996; 62:631-636. 9. Naser S.A, Schwartz D., Shafran I. Isolation of Mycobacterium avium subsp paratuberculosis from breast milk of Crohn’s disease patients. AJG,2000;95:1094-1095. 10. Hermon-Taylor J., Quirke P. Mycobacterium avium subspecies paratuberculosis is a cause of Crohn’s disease. Gut 2001; 49:755-760. 11. Greenstein R.J. Is Crohn’s disease caused by Mycobacterium? Comparisons with leprosy, tuberculosis, and Johne’s disease. The Lancet infectious diseases 2003; 3:507-514. 12. Gross M.T, Sanderson J.D, Tizard M.L.V, Hermon-Taylor J.,.El-Zaatari F.A.K.,Maresich D.C,.Graham D.Y. Polymerase chain reaction detection of Mycobacterium paratuberculosis and Mycobacterium avium subsp silvaticum in long term cultures from Crohn’s disease and control tissues. Gut, 1992; 33:1209-1213. 13. Bull T.J,.McMinn E.J, Sidi-Boumedine K.,.Skull A., Durkin D., Neild P. et al. Detection and verification of Mycobacterium avium subsp. Paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn’s disease. JCM, 2003; 41 :2915-2923. 14. Rowbotham D.S, Mapstone N.P, Trejdosiewicz L.K ,.Howdle P.D, Quirke P., Mycobacterium paratuberculosis DNA not detected in Crohn’s disease tissue by fluorescent polymerase chain reaction. Gut ,1995; 37:660-667. 15. Fidler H.M, Thurell W., McI Johnson N.,.Rook G.A,.McFadden J.J. Specific detection of Mycobacterium paratuberculosis DNA associated with granulomatous tissue in Crohn’s disease. Gut, 1994;35:506-510. 16. Suenaga K., Yokoyama Y., Okazaki K.,.Yamamoto Y. Mycobacteria in the intestine of Japanese patients with inflammatory bowel disease. AJG ,1995;90: 76-80. 17. Dumonceau J.M,. Gossum A.V, Adler M., .Fonteyene P.A,.Vooren J.P.V, Deviere J. No Mycobacterium paratuberculosis found in Crohn’s disease using the Polymerase Chain Reaction. Digestive Diseases and Sciences, 1996; 41: 421-426. 18. Clarkston W.K,.Presti M.E,.Petersen P.F,.Zachary P.E, Fan W.X,.Leonardi C.L et al., Role of Mycobacterium paratuberculosis in Crohn’s disease. Dis Colon Rectum, 1998; 41: 195-199. 19. Sanderson J.D, Moss M.T, Tizard M.L.V,.Hermon-Taylor J. Mycobacterium paratuberculosis DNA in Crohn’s disease tissue. Gut, 1992; 33:890-896. 20. DellÍsola B., Poyart C., Goulet O. ,.Mougenot J.F, Sadoun-Journo E., Brousse N. et al. Detection of Mycobacterium paratuberculosis by Polymerase Chain Reaction in children with Crohn’s disease. JID ,1994;169:449-451. 21. Hulten K.,.El-Zimaity H.M.T,.Karttunen T.J,.Almashrahrawi A., Schwartz M.R, Graham D.Y et al. Detection of Mycobacterium avium subspecies paratuberculosis in Crohn’s disease tissues by In Situ Hybridization. AJG, 2001; 96:1529-1535. 22. Bernstein C.N, Nayar G., Hamel A., Blanchard J.F, Study of animal-borne infections in the mucosae of patients with inflammatory bowel disease and population —based controls. JCM , 2003 ;41, :4986-4990. 23. Bernstein C.N,.Blanchard J.F, Rawsthorne P., Collins M. Population-based case control study of seroprevalence of Mycobacterium paratuberculosis in patients with Crohn’s disease and ulcerative colitis. J. clin. Microbiol., 2004; 42:1129-1135. 24. Stainsby K.J, Lowes J.R, Allan R.N,.Ibbotson J.P, Antibodies to Mycobacterium paratuberculosis and nine species of environmental mycobacteria in Crohn’s disease and control subjects. Gut, 1993; 34:371-374. 25. Borody T.J, Leis S.,.Warren E.F, Surace R. Treatments of severe Crohn’s disease using antimycobacterial triple therapy-approaching a cure? Digest. Liver Dis. 2002; 34:29-38. 35. Jarnerot G., Rolny P., Wickbom G., Alemayehu G. Antimycobacterial therapy ineffective in Crohn’s disease after a year. The Lancet, 1989; 164-165. 36. Schultz M.G, Reader H.L, Hersh T., Riepe S. Remission of Crohn’s disease with antimycobacterial chemotherapy. The Lancet, 1987; 1391-1392. 37. Graham D.Y, Al-Assi M.T, Robinson M. Prolonged remission in Crohn’s disease following therapy for Mycobaterium paratuberculosis. Gastroenterology,1995;108:A826. 38. Woodgame R.W, Kimball k., Akram S, Graham D.Y, Ou C.N. Randomized controlled trial of clarithromycin and ethambutol in the treatment of Crohn’s disease. Gastroenterology,1999;116(suppl):A725. 39. Borody T J, Pearce L,Bampton P A ,Leis S. Treatment of severe Crohn’s disease using rifabutin-macrolide-clofazimine combination:interim report. Gastroenterology,1998;114:A938. 40. Behr M.A, Semret M., Poon A., Schurr E. Crohn’s disease, mycobacteria, and NOD2. The Lancet infectious diseases, 2004; 4:136-137. 41. Greenstein R.J, Collins M.T. Emerging pathogens: is Mycobacterium avium subspecies paratuberculosis zoonotic? The Lancet ,2004;364:396-397. 42. Borgaonkar M.R, MacIntosh D.G, Fardy M.J.A meta-analysis of antimycobacterial therapy for Crohn’s disease. AJG,2000;95:725-728. 43. Hulten K, Almashhrawi A., El-Zaatari F.A.K, Graham D.Y. Antibacterial therapy for Crohn’s disease: a review emphasizing therapy directed against mycobacteria. Digestive diseases and Sciences, 2000; 45: 445-456.