Year 11 Chemistry Revision

Chemicals
The Periodic Table – Revision
What are atoms like?
An atom is a (positive) nucleus surrounded by (negative) electrons, giving the atom, an overall neutral charge.
The nucleus is made up of protons (positive) and neutrons (neutral)
The atom is neutral because the number of electrons = number of protons
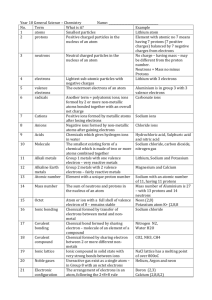
Particle
Electron
Proton
Neutron
Mass number
Relative Charge
-1
+1
0
12
Relative Mass
0.0005 (zero)
1
1
C
Atomic number 6
The atomic number is the number of protons in an atom (and remember, n˚ of protons = n˚ of electrons).
The mass number = number of protons + number of neutrons
Isotopes are varieties of an element that have the same atomic number but different mass numbers.
There are just over 100 elements and an element is a substance which
cannot be broken down chemically
contains the came type of atom.
A compound is a substance that contains at least two elements, chemically combined.
Elements in the periodic table are arranged in ascending atomic number.
Electrons occupy the space around the nucleus and are arranged in shells.
Higher – Need to work out the electronic structure of the first 20 elements in the periodic table e.g. calcium is 2.8.8.2.
How atoms combine – Ionic bonding
An ion is a charged atom or group of atoms.
A positive ion is formed when electrons are lost e.g. 2+ ions form by the loss of 2 electrons.
A negative ion is formed when electrons are gained e.g. 2- ions form by the gain of 2 electrons.
Ionic bonding
-
A metal and a non metal combine
-
Electrons are transferred forming ions
-
The ions are then attracted to one another.
Sodium chloride
has a high melting point (there is a strong attraction between positive
and negative ions)
-
Dissolves in water
-
When solid, does not conduct electricity (the ions cannot move)
-
Conducts when is in solution (Ions can move)
-
Conducts when is molten (Ions can move).
Magnesium oxide
has a very high melting point
when solid does not conduct electricity
-
Conducts when molten.
Higher: Sodium chloride and magnesium oxide are giant ionic lattices in which positive ions are electrostatically attracted to negative ions.
Higher -
Covalent bonding and the structure of the periodic table
A molecule is two or more atoms bonded together.
You need to be able to state the number of atoms and how many different types of atoms, there are given a molecules display formula.
There are two types of bonding, ionic and covalent.
Covalent bonding
non metals
sharing of electrons
Higher : Describe the formation for simple molecules containing single and double bonds by the “dot and cross” model limited to the molecules H
2
, C
12
, CH
4
, CO
2
, H
2
O.
Carbon dioxide (gas) and water (liquid)have a low melting point and don’t conduct electricity.
They are simple molecules with weak intermolecular forces between molecules which means they are easily broken and therefore have a low melting point.
They also don’t have free electrons so don’t conduct electricity.
Groups in a periodic table are vertical columns and all the elements in each group have similar properties.
An element’s group number is the same as the number of electrons in the outer shell.
A period of elements are all the elements in a horizontal row of the periodic table.
The period that an element belongs in corresponds to the number of shells in the electronic structure.
The Group 1 Elements
Group 1 metals are known at the alkali metals (sodium, lithium and potassium)
Group 1 metals react vigorously with water and are stored under water because they react with water and air.
When they react with water,
hydrogen is formed
-
The hydroxide of the metal is formed, e.g. sodium hydroxide, which is an alkali.
-
Reactivity increases down the group
-
Potassium burns with a lilac flame.
-
E.g. Sodium + Water Sodium hydroxide + Hydrogen
(same for potassium and lithium)
-
2Na + 2H
2
O 2NaOH + H
2
(Same for potassium, K, and Lithium, Li)
Reactivity with water – Potassium (most reactive), sodium, lithium (least reactive).
Predict the properties of alkali metals e.g
-
Reactivity of rubidium with water
-
The physical properties of caesium given information about the other alkali metals.
Group 1 metals have 1 outer electron.
Group 1 metals have similar properties due to the one outer electron.
Alkali metals have similar properties because when they react an atom loses one electron to form a positive ion with a stable electronic structure, E.g.
Na - 1e Na 1+
The more reactive the alkali metal, the easier it is for an atom to lose one electron.
The loss of electrons is Oxidation (O.I.L)
Flame tests
-
Sodium – Orange
-
Lithium – Red
-
Potassium – Lilac
To carry out a flame test you,
-
Use a moistened flame test wire
-
Dip the wire into a solid sample
-
Place the flame test wire into a blue Bunsen flame.
The Group 7 Elements
Group 7 elements are known as halogens (fluorine, chlorine, bromine and iodine).
Appearance at room temperature
chlorine is a green gas
bromine is an orange liquid
iodine is a grey solid
Uses
chlorine – sterilise water, make pesticides and plastics
-
Iodine – to sterilise wounds
Halogens react vigorously with alkali metals to form metal halides, e.g. sodium chloride.
You need to be able to construct a word equation (and a balanced symbol equation – higher) for the reaction of a halogen with an alkali metal and identify the halide formed
- e.g. Sodium + Chlorine Sodium chloride (sodium chloride is
2Na + Cl
2
2NaCl halide formed)
Reactivity of halogens – Fluorine (most reactive), chlorine, bromine, iodine (least reactive)
Reactivity decreases as you go down the group.
Halogens can displace each other from halides, depending on their reactivity (more reactive halogens with displace less reactive ones, e.g. Chlorine displaces bromide in sodium bromide to form sodium chloride and bromine).
-
Chlorine displaces bromides and iodides
-
Bromine displaces iodides
-
Iodine does not displace any.
Construct a word equation for the reaction between a halogen and a metal halide e.g. Sodium bromide + chlorine Sodium chloride + Bromine
Construct a balanced symbols equation for the reaction between a halogen and a metal halide e.g. 2NaBr + Cl
2
2NaCl + Br
2
Predict the properties of fluorine and astatine given the properties of the other halogens e.g.
physical properties
displacement reactions
Group 7 elements have similar properties because they have seven electrons in the outer shell.
Halogens have similar properties because when they react they gain one electron to form a negative ion with a stable electronic structure.
Cl + 1e Cl 1-
The more reactive the halogen the easier it is for an atom to gain an electron.
The gaining of electrons is Reduction (R.I.G)
Explain why it is a reduction from its ionic equation e.g. Cl
2
+ 2Br 12Cl 1 + Br
2
(Chlorine is Reduction - gaining electrons, bromine is Oxidation - losing electrons)
Electrolysis
Electrolysis is the decomposition (break down) of a liquid by using an electric current.
During electrolysis
the anode is the positive electrode
the cathode is the negative electrode
anions are negative ions and are attracted to the cathode
an electrolyte is the liquid which conducts electricity
-
Anions are negative ions (negative ions get attracted to the positive electrode)
-
Cations are positive ions (positive ions get attracted to the negative electrode)
Sulphuric acid can be broken down by electrolysis to form oxygen (made at the anode) and hydrogen (made at the cathode).
At the cathode - 2H + + 2e H
2
At the anode - 4OH - 4e 2H
2
O + O
2
Test for hydrogen – ‘squeaky pop’ test – light it using a lit splint, if it pops, its hydrogen
Test for oxygen – Oxygen relights a glowing splint.
Aluminium is extracted from its ore (Bauxite) using electrolysis.
Electrolysis of Aluminium
- The aluminium oxide is molten (as it is insoluble)
- Oxygen is formed at the graphite anode
- The anodes are gradually worn away, when the oxygen reacts to form carbon dioxide
- Aluminium is formed at the graphite cathode
- The process requires a lot of electricity (which is why aluminium is expensive)
Aluminium oxide Aluminium + Oxygen
Electrode reactions
- Cathode – Al 3+ + 3e -
- Anode – 2O 2O
2
Al
+ 4e -
Aluminium oxide has a very high melting point so cryolite is added to lower the melting point.
Transition Elements
Transition elements are metals with typical metallic properties and are found in the middle of the periodic table, e.g. copper and iron.
Transition elements have coloured compounds
- Copper compounds are blue
- Iron (II) compounds are light green
- Iron (III) compounds are orange/brown
Transition elements and their compounds are often used as catalysts
- Iron is used in the Haber process
- Nickel is used in the manufacture of margarine
Thermal decomposition is a reaction in which a substance is broken down into at least two other substances by heat.
In the thermal decomposition of Iron carbonate (FeCO
3
), copper carbonate (CuCO
3
),
Manganese carbonate (MnCO
3
) and zinc carbonate (ZnCO
3
),
- The metal oxide and carbon dioxide is formed
- E.g. Copper Carbonate
- A colour change occurs
Copper oxide + Carbon dioxide
- CuCO
3
CuO + CO
2
A precipitation is a reaction between solutions that forms an insoluble solid.
Sodium hydroxide can be added to transition metal ion solution to identify the metal ion present (the colour of the precipitate can be used to identify)
- Cu 2+ gives a blue solid
- Fe 2+ gives a grey/green solid
- Fe 3+ gives an orange/brown solid
- Cu 2+ + OH Cu(OH)
2
- Fe 2+ + OH -
- Fe 3+ + OH -
Fe(OH)
2
Fe(OH)
3
Metal structure and properties
Iron is used to make steel and to make cars because it is strong.
Copper is used to make brass and to make electrical wiring because it is a good electrical conductor.
Physical properties of metals
- Lustrous, hard and high density
- High tensile strength
- High melting and boiling points
- Good conductors of heat and electricity.
The particles in a metal are held together by strong metallic bonds which is why they have high melting and boiling points.
Metallic bonds are the strong electrostatic attraction between a sea of delocalised electrons (negative) and close packed positive metal ions.
The high melting and boiling points are due to this strong attraction, which has to be overcome.
Metals have a structure which contains crystals. The particles are close together and have a regular arrangement.
At low temperatures some metals can be superconductors.
When metals conduct electricity, the delocalised electrons move easily.
Superconductors are materials that conduct electricity with little or no resistance.
Benefits of super conductors
- loss free power transmission
- super-fast electronic circuits
- Powerful electromagnets
Drawbacks of superconductors
- only work at very low temperatures
- there is a need to develop superconductors that work at 20 ºC
Acids and alkalis
Acids have a pH of less than 7, alkalis have a pH of more than 7 and solutions with pH of 7 are neutral.
We can use universal indicator to estimate the pH of a solution – universal indicator changes the colour of the solution, which can then be compared to the pH scale.
When you add an alkali the pH increases, when you add an acid, the pH decreases.
A base is a substance that neutralises an acid. Some bases dissolve in water to form and alkali.
A neutralisation reaction is
- acid + base salt + water
Acids in solution contain hydrogen ions.
Alkalis in solution contain hydroxide ions
Ionic equation for neutralisation is –
H + + OH H
2
O
An acid can be neutralised by either a base or an alkali and vice versa.
Metal oxides and metal hydroxides neutralise acids because they are bases.
Carbonates (e.g. sodium carbonate) neutralise acids to give mater, a salt and carbon dioxide
The salt produced depends on the base and the acid,
- Hydrochloric acid produces chlorides (e.g. sodium chloride)
- Sulphuric acid produces sulphates (e.g. sodium sulphate)
- Nitric acid produces nitrates (e.g. sodium nitrate)
Higher – construct word equations to show an acid being neutralised by bases or carbonates (without given the names of products)
Higher – construct balanced symbol equations to show an acid being neutralised by a base or carbonate, using only the following;
- Sulphuric acid – H
2
SO
4
- Nitric acid – HNO
3
- Hydrochloric acid – HCl
- Ammonia – NH
3
- Potassium hydroxide – KOH
- Sodium hydroxide – NaOH
- Copper oxide – CuO
- Sodium carbonate – Na
2
CO
3
- Calcium carbonate – CaCO
3
Reacting masses
You need to be able to calculate the relative formula mass of a substance, given its formula
(find their relative atomic mass on a periodic table and add them up – E.g. Water, H
2
0 =
1+1+16 = 18
The more starting materials you have (reactants), the more product would be formed.
The total mass of reactants = total amount of product
You need to be able to calculate how much product/reactant is made/used from an equation.
Percentage yield is a way of comparing the amount of product made (actual yield) to the amount expected (predicted yield)
- 100% yield means that no product has been lost
- 0% yield means that no product has been made
Possible reasons why the percentage yield of a product is less than 100%
- loss in filtration
- loss in evaporation
- loss in transferring liquids (some may remain in the beaker etc)
- loss in heating (boil off etc)
Percentage yield = actual yield x 100
Predicted yield
Fertilisers and crop yield
Fertilisers make crops grow faster and bigger.
The plants absorb minerals through their roots. To do this, the fertiliser must first be dissolved in water.
Fertilisers increase the crop yield.
- They replace essential elements used by a previous crop or provides extra essential elements.
- More nitrogen gets incorporated into plant protein so increased growth.
Eutrophication is a form of pollution.
- Rain water dissolves the fertiliser, which runs off into rivers and streams.
- This increases the concentration of nitrates and phosphates in the river.
- Microscopic water plants called algae grow rapidly (algal bloom)
- The algae blocks the sunlight from reaching other plants.
- The plants die due to lack of sunlight and nutrients.
- Aerobic bacteria feed on dead plants, using up the oxygen in the water.
- Fish and other water animals die due to lack of oxygen.
Fertilisers are chemicals which provide plants with essential chemical elements.
Nitrogen, potassium and phosphorus are the three essential elements for plant growth.
Fertilisers are made by a neutralisation reaction (you need to be able to label the apparatus).
Nitrogenous fertilisers include
- ammonium nitrate – made from ammonia and nitric acid.
- ammonium phosphate – made from ammonia and phosphoric acid.
- ammonium sulphate – made from ammonia and sulphuric acid.
To make a fertiliser
- Measure our 25cm 3 of base using a measuring cylinder and add a few drops of litmus solution.
- Using a pipette, add acid dropwise until the solution just turns red.
- Count the number of cm 3 used.
- Wash out the beaker, measure out another 25cm 3 of base and add the same amount of acid that was required to neutralise the base.
- Pour the solution into an evaporating dish, place on a tripod and gauze and heat carefully to concentrate the solution. When it has reduced by a half, place on the windowsill to remove the remaining water.
Haber Process and costs
Ammonia is made from nitrogen and hydrogen
The nitrogen needed to make ammonia is obtained from the air and the hydrogen needed often comes from the cracking of oil fractions or natural gas.
Nitrogen + Hydrogen
N
2
+ 3H
2
Ammonia
2NH
3
means that it is a reversible reaction i.e. the reaction can go in both directions.
Nitrogen and hydrogen are reacted together under carefully controlled conditions
-
High pressure (150 atmospheres) – increases the % yield
-
Temperature about 450°C – high temperature decreases the % yield
but increases the rate of reaction, so 450°C is used as a compromise
-
Iron catalyst – increases the rate of reaction but does not affect the
yield
This gives a yield of 15%, any unreacted nitrogen and hydrogen is recycled in a continuous process (they get sent around again)
The cost of making a new substance depends on
price of energy (gas and electricity)
-
Cost of starting material
-
Wages (labour cost)
-
Equipment (Plant costs)
-
How quickly the new substance can be made (cost of catalyst)
Certain factors affect the cost of making a new substance
-
The higher the pressure, the higher the plant cost
-
The higher the temperature the higher the energy cost
-
Catalysts reduce costs by increasing the rate of reaction
-
When unreacted starting materials are recycled, costs are reduced
-
Automation (systems run by computers) reduces the wages bill
Economic considerations determine the conditions used , in the manufacturing of chemicals
-
The rate must be high enough to give a sufficient daily yield of the product
-
Percentage yield must be high enough to give a sufficient daily yield of product
-
A low percentage yield can be accepted if the reaction can be repeated many times with recycled started materials
-
Optimum conditions used that give the lowest cost rather than the fastest reaction or highest percentage yield
Uses of ammonia
-
Manufacture of fertilisers
-
Manufacture of nitric acids
-
In cleaning fluids
Solvent – A solvent is a liquid which dissolves a solid. Different solvents dissolve different substances.
Solute – A solute is the thing that is dissolved in a solvent, e.g. salty water – water is the solvent, salt is the solute.
Soluble – Means it can dissolve.
Insoluble – Means it cannot dissolve.
Dry cleaning is a process used to clean clothes that does not involve water. Instead a solvent that isn’t the water is used to remove stains which don’t dissolve in water. Water molecules have small positive and negative charges which only attract substances which also have charges (molecules with no charge are not attracted). Oil and grease have no charge but form intermolecular forces of attraction between the uncharged oil and grease
and the uncharged molecule of an organic solvent (used in dry cleaning).
Many detergents are salts, made by the neutralisation of acids with alkalis.
Batch or continuous
Ammonia is an example of a continuous process – any remaining unreacted reactants are sent back around and is run 24 hours a day (large scale).
Speciality chemicals such as medicines and pharmaceutical drugs are often made in a batch process (small scale).
Factors which affect the cost of making and developing a medicine or pharmaceutical drugs include
- Research and testing
- Labour costs – often more labour intensive, less automation possible
- Energy costs
- Raw materials – may be rare and/or involve expensive extraction from plants
- Time taken for development – May take many years
- Marketing – Legislative demands
There are several economic considerations, which determine the development of new drugs,
- The research and development time and associate labour costs
- Time required to meet legal requirements including timescale for testing, human trials.
- Anticipated demand for new product
- Length of pay back time for initial investment.
The raw materials for speciality chemicals such as pharmaceuticals can either be made synthetically or extracted from plants.
Extraction from plant
- Plant is crushed
- It is then dissolved in a suitable solvent
- Filtered
- Purified
- The solvent is evaporated off, leaving the drug
- Chromatography is used to test the purity
Nanochemistry
Chemistry normally works with chemicals on a large scale. Nanochemistry, however, uses materials on a very small scale, the tiny particles are called nanoparticles.
Nanoparticles have different properties from bulk chemicals.
Three forms (allotropes) of carbon are
- Diamond
- Graphite
- Buckminster fullerene (bucky balls)
Diamond
- Physical properties - lustrous, colourless and clear (transparent)
- hard and has a high melting point (has many strong covalent bonds)
- insoluble in water
- does not conduct electricity (because it has no free electrons)
- Uses - cutting tools - because it is very hard and has a high melting point
- jewellery - because it is lustrous and colourless
Graphite
- Physical properties -black, lustrous and opaque
- Slippery (because layers of carbon atoms are weakly held together
can easily slide over each other)
- insoluble in water
- high melting point (because there are many strong covalent bonds)
- conducts electricity (because delocalised electrons can move ` about)
- Uses - pencil leads – because it is slippery and black
- Lubricants – because it is slippery
- Electrodes – because it conducts electricity and has a high melting point
Buckminster fullerene (C
60
)
- physical properties - black solid
-deep red in solution in petrol
- Uses - They can be used to ‘cage’ other molecules, as a drug delivery system
Buckminster fullerenes can be joined together to make nanotubes.
Nanotubes – properties – Strong
- Conduct electricity
- Uses - semiconductors in electrical circuits
- industrial catalysts (the catalyst is attached to the nanotubes and mean that there is a large surface area)
- reinforce graphite in tennis rackets
It may be possible to use nanoparticles as miniature factories producing chemical products
– this is known as molecular manufacturing. Nanoparticles can be used to assemble a product, molecule by molecule, each nanoparticles bringing a different part to be positioned precisely in the assembly of a complete molecule – known as positional chemistry.
Another possibility is to start with a larger structure and remove part of it bit by bit.
How pure is our water?
Water is found in
lakes
rivers
aquifers
reservoir
Water is an important industrial resource and is used as
a cheap raw material
a coolant
a solvent
It is important to conserve water to prevent shortage.
Pollutants found in domestic water supplies
nitrate residues from fertiliser run off
lead compounds from old lead pipes
pesticides from spraying near to a water resource
Before water is purified, it contains
dissolved salts and minerals
microbes
pollutants
insoluble minerals
Chlorination is used to kill the microbes in water.
Purification involves
1.
Sedimentation – large solids fall to the bottom of the sedimentation tank
2.
Filtration – a filter made up of grit and course and fine sand, traps finer solids such as clay, which is too small to settle out in the sedimentation tank.
3.
Chlorination – chlorine is added in small amounts to kill the microbes
Some soluble substances are not removed from water purification, which can be poisonous.
In hot countries and on boats and yachts, sea water is distilled, leaving the salt behind. On
a large scale, distillation uses a lot of energy.
Barium chloride solution is used to test for sulphate ions, producing a white precipitate.
E.g. Barium chloride + sodium sulphate Barium sulphate + sodium chloride
BaCl
2
+ Na
2
SO
4
BaSO
4
+ 2NaCl
Higher – need to be able to write balanced equations for the reaction between barium chloride and sulphates, when given the appropriate formulae.
Silver nitrate solution can be used to test for halides ions (compounds containing halogens)
-
Chloride ions give a white precipitate
E.g. Sodium Chloride + Silver Nitrate Silver chloride + Sodium nitrate
NaCl + AgNO
3
AgCl + NaNO
3
Higher – need to be able to write balanced equations for the reaction between silver nitrate and chlorides, when given the appropriate formulae.
-
Bromide ions give a cream precipitate
E.g. Sodium bromide + Silver nitrate Silver bromide + Sodium nitrate
-
Iodide ions give a yellow precipitate
E.g. Potassium iodide + Silver nitrate Silver iodide + Potassium nitrate
These are all precipitate reactions