Metal Reactivity Series: Oxygen, Water, & Ion Reactions
advertisement
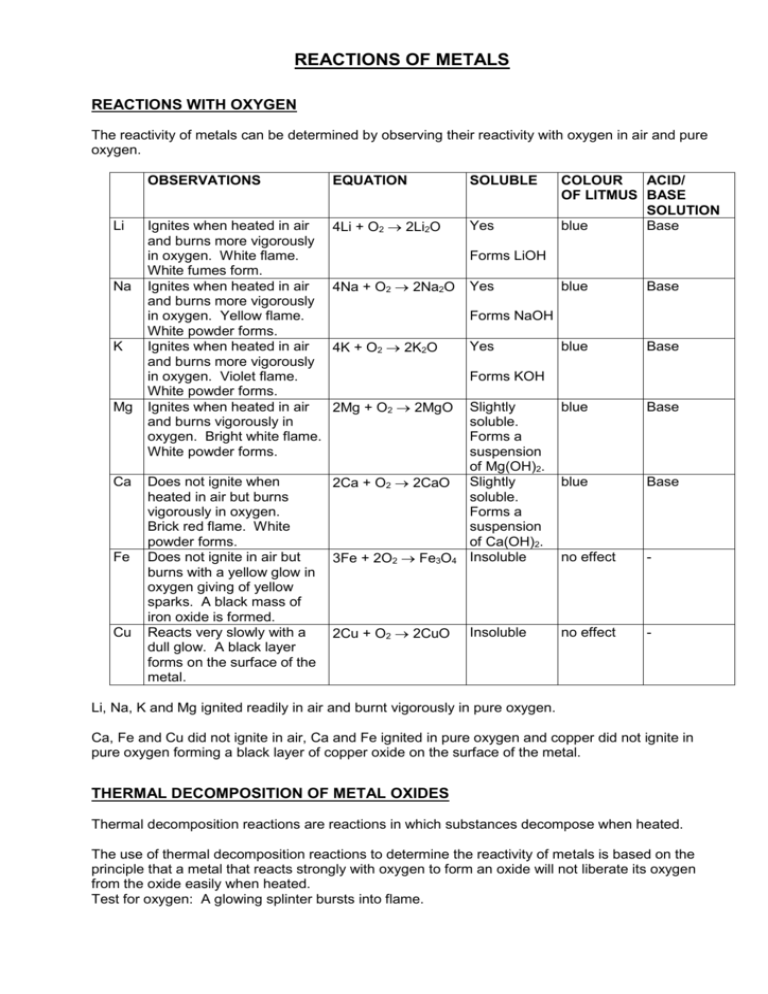
REACTIONS OF METALS REACTIONS WITH OXYGEN The reactivity of metals can be determined by observing their reactivity with oxygen in air and pure oxygen. Li Na K Mg Ca Fe Cu OBSERVATIONS EQUATION SOLUBLE Ignites when heated in air and burns more vigorously in oxygen. White flame. White fumes form. Ignites when heated in air and burns more vigorously in oxygen. Yellow flame. White powder forms. Ignites when heated in air and burns more vigorously in oxygen. Violet flame. White powder forms. Ignites when heated in air and burns vigorously in oxygen. Bright white flame. White powder forms. 4Li + O2 2Li2O Yes Does not ignite when heated in air but burns vigorously in oxygen. Brick red flame. White powder forms. Does not ignite in air but burns with a yellow glow in oxygen giving of yellow sparks. A black mass of iron oxide is formed. Reacts very slowly with a dull glow. A black layer forms on the surface of the metal. 2Ca + O2 2CaO COLOUR ACID/ OF LITMUS BASE SOLUTION blue Base Forms LiOH 4Na + O2 2Na2O Yes blue Base blue Base blue Base blue Base 3Fe + 2O2 Fe3O4 Slightly soluble. Forms a suspension of Mg(OH)2. Slightly soluble. Forms a suspension of Ca(OH)2. Insoluble no effect - 2Cu + O2 2CuO Insoluble no effect - Forms NaOH 4K + O2 2K2O Yes Forms KOH 2Mg + O2 2MgO Li, Na, K and Mg ignited readily in air and burnt vigorously in pure oxygen. Ca, Fe and Cu did not ignite in air, Ca and Fe ignited in pure oxygen and copper did not ignite in pure oxygen forming a black layer of copper oxide on the surface of the metal. THERMAL DECOMPOSITION OF METAL OXIDES Thermal decomposition reactions are reactions in which substances decompose when heated. The use of thermal decomposition reactions to determine the reactivity of metals is based on the principle that a metal that reacts strongly with oxygen to form an oxide will not liberate its oxygen from the oxide easily when heated. Test for oxygen: A glowing splinter bursts into flame. OXIDE OBSERVATION EQUATION CuO Does not liberate oxygen. - MgO Does not liberate oxygen. - PbO2 HgO The brown colour of lead(IV) oxide changes to the yellow colour of lead(II) oxide. Small quantities of oxygen are given off. Droplets of mercury form at the cool mouth of the test tube. Oxygen easily liberated. 2PbO2 (brown) 2PbO (yellow) + O2 2HgO 2Hg + O2 Since Cu and Mg did not give up their oxygen, they are the most reactive of the four elements. Pb and Hg are less reactive with Hg being the least reactive because it gave up its oxygen easily. REACTIONS WITH WATER The reactivity of metals can also be determined by observing their reactions with water. Metals react with water to produce hydrogen. The rate at which hydrogen is produced indicates the reactivity of the metal. Test for hydrogen: A small explosion occurs when a match is held at the mouth of the test tube. Alkali metals are stored under paraffin to prevent oxidation. Reactions with cold water: Li Na K Ca Mg Fe Cu OBSERVATIONS Floats, does not melt, moves around the surface and gives off hydrogen gas. Floats, melts, moves around the surface and gives off hydrogen gas. Floats, melts, moves around the surface and gives off hydrogen gas that ignites and burns with a blue flame. Calcium sinks and bubbles of hydrogen are given off. The solution becomes cloudy due to the Ca(OH)2 produced that is slightly soluble in water. No reaction No reaction No reaction EQUATION 2Li + 2H2O 2LiOH + H2 2Na + 2H2O 2NaOH + H2 2K + 2H2O 2KOH + H2 Ca + 2H2O Ca(OH)2 + H2 - The metals that did not react with cold water are then placed in warm water: Mg Fe Cu OBSERVATIONS Magnesium sinks and bubbles of hydrogen are given off. The solution becomes cloudy due to the Mg(OH)2 produced that is slightly soluble in water. No reaction No reaction EQUATION Mg + 2H2O Mg(OH)2 + H2 - The metals that did not react with warm water are then reacted with steam: Fe Cu OBSERVATIONS Glows brightly giving off hydrogen. Black iron(IV) oxide is formed. No reaction EQUATION 3Fe + 4H2O Fe3O4 + 2H2 - REACTIONS OF METALS WITH METAL IONS In the above reactions, metals have been in competition with hydrogen for the possession of oxygen in water. Since all the metals except copper reacted with the oxygen in water, it can be concluded that hydrogen is more reactive than copper. The same principle can be applied to reactions between metals and metal ions. A metal can displace from solution any other metal ion that is lower in the reactivity series, except when the metal reacts with water. Metals cannot displace their own ions. The following table shows the displacement of metal ions from solution by metals: Mg Zn Fe Cu MgSO4 (Mg2+) x x x x ZnSO4 (Zn2+) x x x FeSO4 (Fe2+) x x CuSO4 (Cu2+) x The following reactions occurred in the above table: Mg + ZnSO4 MgSO4 + Zn Mg + FeSO4 MgSO4 + Fe Mg + CuSO4 MgSO4 + Cu Zn + FeSO4 ZnSO4 + Fe Zn + CuSO4 ZnSO4 + Cu Fe + CuSO4 FeSO4 + Cu The above metals can be arranged in order of increasing reactivity as follows: Cu Fe Zn Mg REACTIVITY SERIES From observations of reactions between metals and oxygen, thermal decomposition of oxides, reactions between metals and water, and the displacement of metal ions from solution, metals can be placed in order of increasing reactivity in the reactivity series. K Na Li Mg Ca Zn Fe Pb (H) Cu Hg Ag









