Single Replacement Reactions Lab Report
advertisement

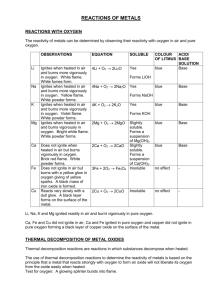
Single Replacement Reactions Background Information: In nature, elements can occur either free, meaning uncombined with other elements, or chemically combined in a compound. The tendency of a particular element to combine with other substances is a measure of the activity of the element. The more active an element is, the more likely it is to combine. In a single replacement reaction, an uncombined element replaces a less active element that is combined in a compound. The less active element is then freed from the compound. For example: Zn (s) + CuSO4 (aq) → Cu (s) + ZnSO4 (aq) solid zinc reacts with aqueous copper (II) sulfate to produce copper metal and aqueous zinc sulfate Zinc replaces the less active copper, combines with sulfate, and frees the copper from the compound. In this investigation, you will observe how various metals undergo single replacement reactions when placed in acid. If the metal is more active than the hydrogen in the acid, it will replace the hydrogen and hydrogen gas will be released. You will also compare the activity of the metals to the activity series of metals. Objectives: 1. To observe the chemical reactions between metals in a dilute acid. 2. To compare and contrast the degree of chemical reactivity among the metals. 3. To compare the activity of the metals to the known activity series. Hypothesis: Determine the expected activity of the metals from most reactive to least reactive. Procedure: a. ***Caution! - This lab involves handling glassware and hydrochloric acid (HCl), which can cause damage to eyes, skin, and clothing. If you come in contact with it - rinse immediately with excess water and inform the instructor. Wear safety goggles and closed toed shoes throughout the entirety of the lab procedure.*** b. Label five test tubes - each with the name of one of the metals (zinc, aluminum, copper, iron and magnesium) if this has not been done already. c. Following your instructor’s demonstration - carefully place about 3 mL of hydrochloric acid into each of the test tubes in the test tube rack. d. One at a time, place a small scoop of the appropriate metal in each test tube. Record your observations in Table 1. e. When finished, place any waste in the proper waste container in the fume hood. Then rinse the inside and outside of each test tube with large amounts of water. Invert each tube in the test tube rack to dry and then wipe down the entire lab bench. Data: Table 1:______________________________ Reactivity 1 (Most Active) 5 (Least Active) Before Reaction During and After Reaction Zinc Aluminum Copper Iron Magnesium Results: Report the expected reactivity trend (hypothesis) and the experimental reactivity trend Sentence format Conclusion: Explain the type of reaction that occurred between the acid and metals. Compare and contrast the degree of chemical reactivity among the metals. o How did you decide? Did the lab results support the hypothesis? Post Lab Questions: 1. The products of the reaction between Zn and HCl are ZnCl2 and H2. Write a balanced equation for this reaction. 2. Identify two elements besides copper that will not replace hydrogen in when reacted with an acid. Justify. 3. If hydrogen was replaced by a more active metal, hydrogen gas would have been produced. What did you observe in the lab if a gas was produced? 4. Balance the following single replacement reactions: a. ____Mg + ____HCl → ____MgCl2 + ____H2 b. ____Zn + ____H2SO4 → ____ZnSO4 + ____H2 c. ____Cl2 + ____NaBr → ____NaCl + ____Br2 d. ____HgO + ____Cl2 → ____HgCl2 + ____O2 e. ____H2O + ____Fe → ____Fe2O3 + ____H2 RST: In this lab, you observed several chemical reactions and the degree of reactivity of various metals. You will analyze your data and results (Source A), section 9.2 in the text (Source B), and Figure 1 below (Source C) to gather information and formulate a written argument that justifies or refutes the following statement: “In single replacement reactions, atoms change identity.” Figure 1: Activity Series of Metals College Prep Chemistry Core Lab #13 50 points Single Replacement Reactions Format 5 points Written in pencil (1) Underline appropriate titles (1) Use appropriate units throughout and significant digits (1) Title all tables, graph, and figures (1) Tables and graphs are drawn with straight edge (1) Introduction / Hypothesis 9 points Restate the objectives and incorporate how the objectives will be met by performing this lab (4) o Discuss the type of reaction involved, including reactants and products. o Discuss how observations will relate to the products of the chemical reactions (bubbles?). o Link any appropriate definitions to the lab, such as activity series. Five elements listed in correct activity order. Data Complete sentence(s) 9 points Complete table appropriately. Results 4 points Report activity of metals determined in data table. (4) Sentence form Conclusion 8 points Address the objective. Support your answer with lab data and results (3) Defend hypothesis. (2) Conclusion quality: well organized and cohesive and contains no mechanical/grammatical errors. (3) o Well organized and cohesive but contains grammatical or mechanic (2) o Somewhat organized but does not contain any errors (2) o Somewhat organized with some errors (1) o Disorganized with errors (0) Post-Lab Questions RST 5 points Use of complete sentences when appropriate. ________ 10 point Thesis statement (2) Comprehension of key ideas and details (3) o Cites convincing evidence to support analysis, showing full comprehension of complex ideas expressed in the texts provided. Development of ideas and organization (2) o Student response addresses the prompt and provides effective development of the claim by using clear and convincing reasoning, details, and evidence. Appropriate integration of source material/information (3) o Student response utilizes information in a way where the sources “speak with one another” are used adequately to support the student’s claims and lab conclusions. College Prep Chemistry Core Lab #14