Where to Find
advertisement

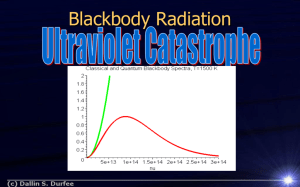
Photoelectric Effect Show the emission of electrons from metals Materials Where to Find Both Electroscopes Static Electricity Kit UV Lamp Shelf 1D Shelf 3E Drawer T3 Set Up Retrieve both of the electroscopes. One has a Zn strip attached and the other has a Mg ribbon in a lightning bolt shape. Only give them the fur and plastic rod, this charges the electroscope by adding electrons. The glass rod will not work because it charges the electroscope by removing electrons (it creates positive static charge). Safety UV short wavelength can be very hazardous. Do not allow it to be shone into eyes. Professor/Lecturer Chemistry This demonstrates the photoelectric effect. In the photoelectric effect, electrons are emitted from matter (metals and non-metallic solids, liquids or gases) as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as "photoelectrons." The work function is related to the amount of energy required to eject electrons from a material. The work functions for Mg and Zn translate to 337 nm and 288 nm respectively. It is important to note that the material must first be charged with an excess of electrons because otherwise the ejected electrons will immediately jump back onto the metals. Presentation Charge up the plastic rod with the fur by rubbing the two together. Use the rod to charge the metals on the electroscopes by sliding the rod across the surface of the metal. The needle on the electroscope should rotate clockwise. Turn on the UV lamp by flipping the switch and then holding down the short wave button and releasing. Shine the UV lamp onto the metals and watch the needle drops back down. Be careful not to touch the lamp to the electroscope because the effect will be ruined since the electrons are transferred to the lamp itself. Misc. notes/Performance Search Items Photoelectric Effect Electroscope Light Electrons UV Lamp Wavelength Suitable for Recitation Reference Wikipedia.