Electroscope Lab
advertisement

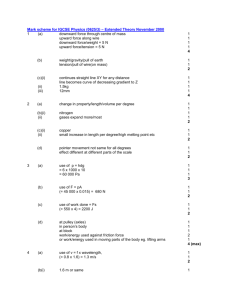
Electroscope Lab Before the Lab What did the leaves look like? Hanging straight down. What is making them stay in that position? Gravity So….What did you observe? What happened to the leaves when you touched the ball after being rubbed on the fabric? They Spread apart What happened to the rods when they were rubbed with the fabric? They became either positively or negatively charged. Observations…. Why did the leaves spread apart? Electrons were transferred off or on the rod. What happened when you touched the ball of the electroscope? The leaves returned to their natural position Electrons transferred to or from the rod to your finger to be neutral Neutral---equal electrons and protons Everything wants to be neutral Observations cont… Which piece of fabric added electrons to the rod? Wool---which gave the white rod a negative charge Which piece of fabric took electrons away from the rod? Silk---which gave the clear rod a positive charge Observations…. How do we know that one piece of fabric made the rod have a positive charge and the other have a negative charge? Part D of the activity The leaves moved closer when the white rod was close to the ball of the electroscope. Started to become Neutral If they were both negative/positive the leaves would have gotten farther apart. Conclusion What is the name of this force that pushes the leaves apart by transfering electrons? Electrostatic Force What can we infer from this lab? Opposite charges attract Like charges repell The charge extends beyond the particle This is the 2nd strongest force in the atom Elements Can’t be divided into any simpler substances. Atoms Smallest particle of an element that still have all the properties of that element. Subatomic Particles Proton—(+) charge, nucleus Neutron—(0) charge, nucleus Electron—(-) charge, electron cloud Elements and Atoms 90 naturally occurring elements 20 synthetic elements What is synthetic? Man made