Supplement file for Lind and Norbeck
advertisement

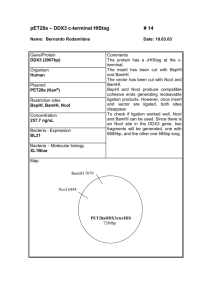
SUPPLEMENT FILE: “A QPCR-based reporter system to study post-transcriptional regulation via the 3’untranslated region of mRNA in Saccharomyces cerevisiae.” Kristina Lind and Joakim Norbeck Cloning and transformation of the 3’UTR-reporter plasmids: A fragment containing 380 bp of the COX17 promotor, upstream of the start codon, was amplified by PCR (with genomic DNA from w303-1A as a template DNA). Primers were designed to incorporate an upstream XhoI site and a BamHI site immediately prior to the start codon. The PCR-fragment was ligated in XhoI/BamHI sites of the centromeric plasmid pRS315 (18), to produce the plasmid p315-COX17p. The 3’UTR of COX17 (333 bp downstream of the last amino acid codon) was amplified by PCR, with primers designed to contain an upstream BamHI-site immediately prior to the STOP-codon and a downstream SacI. The 3’UTR of RPL18B (554 bp downstream of the last amino acid codon) was amplified by PCR, with primers designed to contain an upstream BamHI-site immediately prior to the STOP-codon and a downstream NotI. The 3’UTR of HSP12 (592 bp downstream of the last amino acid codon) was amplified by PCR, with primers designed to contain an upstream BamHI-site immediately prior to the STOP-codon and a downstream SacI. The amplified 3’UTR sequences were digested at the appropriate engineered restriction enzyme sites and ligated into similarly cut p315-COX17p to yield p315-COX17p-BamHI-XXXUTR (with XXX denoting either RPL18B, HSP12 or COX17). Finally, the TAP-tag (16) was amplified from genomic DNA of the NOT5-TAP strain (13), (TAP-UP-primer: GATCGGATCCATGGGTCGACGAATCCCCGGGT and TAPDOWN-primer: GATCGGATCCTCACTGATGATTCGCGTCTAC; It should be noted that in the UP-primer, a substitution of G to A has been included (in bold) to destroy a natural BamHI site. The substitution does not alter the encoded amino acid sequence). This fragment codes for the TAP-tag with an added start methionine codon preceded by a BamHI-site and including the STOP-codon, followed by a BamHI-site. The TAP-tag PCRfragment was digested with BamHI and ligated into the three versions of p315-COX17p- BamHI-XXXUTR, to yield the plasmids p315-COX17p-TAP-XXXUTR (with XXX denoting either RPL18B, HSP12 or COX17). A fragment containing the TAP-tag followed by a stop-codon and the 237 bp ADH1-3’UTR (as found in the TAP-tagged genomic DNA from the NOT5-TAP strain (13)) was amplified using the TAP-UP-primer and an ADH1DOWN-primer (GATCGAGCTCATATTACCCTGTTATCCCTAGCGGATCAGCCGGT). The resulting PCR-fragment was digested with BamHI and SacI, and ligated into similarly cut p315COX17p to yield p315-COX17p-TAP-ADH1UTR. The sequence of the TAP-tag and the 3’UTR sequences have all been verified by sequencing. Plasmids were transformed into strains (BY4742wt, lsm1∆ and pop2∆ (19,20)) as indicated with selection on SC (-leu) 2% (w/v) glucose. A PCR fragment from the primer upstream of the COX17 promotor used together with a primer complementary to the region at the 3’ end of the open reading frame of COX17 (the latter adding a BamHI site immediately preceding the STOP-codon) was ligated between the BamHI and XhoI sites of p315-COX17p-BamHI-XXXUTR (with XXX denoting either COX17 or RPL18B to generate the plasmids 315-COX17p-BH-XXXUTR. The resulting plasmids were cut open with BamHI and a 2xHA-tag was ligated into the site to generate the plasmids 315-COX17-HA-COX17UTR and 315-COX17p-HA-RPL18BUTR. The whole insert was subsequently transferred to pRS316 (18) to generate the plasmids 316COX17-HA-COX17UTR and 316-COX17p-HA-RPL18BUTR which were used for figure 2B. Sequence of 3’UTR reporter plasmid inserts: COX17: Insert sequence from upstream XhoI-site to downstream SacI. Promotor of COX17 is located from XhoI to BamHI, TAP-ORF (Red) is located following the first BamHI-site and ending immediately before the second BamHI-site (the naturally occurring BamHI site in the TAP-tag has been destroyed by a point mutation shown in green). 3’UTR of COX17 is included from BamHI to SacI. ctcgaggtcgacGTCTTCACTGTCACTGATTGTAGGGACAAAGTCCAAATTAGAGTATTTT TTAGTTCCTACCACCATAGTATATATATAAGACGCTGTTGTAGGCACACAGCTCCAATTCG AAACCCAAGTTACCAACGTAAATACTCATACTATCTAAGCATATTCTAACTTACAATTTTC AACCTCATCTCATCGCATTTTTTGAAAATTTTTTTTCAAATCATTACCCGGATAATGTTAT AACCCTACCTATCTCCTTCAAAAAAAGATAAAATGACGATATATAGCGGCGAATCAGAATC TAGTAACCACCTAACTACTTGTCTCTAGTACAACTAAACAAAATTTTAGTGTACACAATCA GATAACTACACAATCAATTATACCCAGGATCCATGGGTCGACGAATCCCCGGGTTAATTAA TCCATGGAAGAGAAGATGGAAAAAGAATTTCATAGCCGTCTCAGCAGCCAACCGCTTTAAG AAAATCTCATCCTCCGGGGCACTTGATTATGATATTCCAACTACTGCTAGCGAGAATTTGT ATTTTCAGGGAGAATTCGGCCTTGCGCAACACGATGAAGCCGTGGACAACAAATTCAACAA AGAACAACAAAACGCGTTCTATGAGATCTTACATTTACCTAACTTAAACGAAGAACAACGA AACGCCTTCATCCAAAGTTTAAAAGATGACCCAAGCCAAAGCGCTAACCTTTTAGCAGAAG CTAAAAAGCTAAATGATGCTCAGGCGCCGAAAGTAGACAACAAATTCAACAAAGAACAACA AAACGCGTTCTATGAGATCTTACATTTACCTAACTTAAACGAAGAACAACGAAACGCCTTC ATCCAAAGTTTAAAAGATGACCCAAGCCAAAGCGCTAACCTTTTAGCAGAAGCTAAAAAGC TAAATGATGCTCAGGCGCCGAAAGTAGACGCGAATCATCAGTGAGGATCCTAGTCTTACTG ACAGTCTGCCGACAACCATTTCTTGTATATATAAGAATAGGTATTCACAAACTATAGTATA TACTACCTGTAAATATGTGCGATGCACAATTAACATTACCTCATCACTACTACACCACTTC TACTGCTAGCACTGTCCTCTTGTGCTTGGCCCCTTAAGAGTGTTCTAAGACCACGTGACCA GAAAAGGACGTATCACGTGACACAAACCTAATAACTTTTTTAGGAAACCCTAAGTCAGGGC ACGCTTGCTTTGTTTCTTTTTTTTCTGGAAAGAACGGAAAAAAAAAAAAGTCTGGGATTAG TTAGTGTATATTAAGTGgagctc RPL18B: Same as the insert for COX17, except that the RPL18B 3’UTR is cloned using BamHI and NotI. ctcgaggtcgacGTCTTCACTGTCACTGATTGTAGGGACAAAGTCCAAATTAGAGTATTTT TTAGTTCCTACCACCATAGTATATATATAAGACGCTGTTGTAGGCACACAGCTCCAATTCG AAACCCAAGTTACCAACGTAAATACTCATACTATCTAAGCATATTCTAACTTACAATTTTC AACCTCATCTCATCGCATTTTTTGAAAATTTTTTTTCAAATCATTACCCGGATAATGTTAT AACCCTACCTATCTCCTTCAAAAAAAGATAAAATGACGATATATAGCGGCGAATCAGAATC TAGTAACCACCTAACTACTTGTCTCTAGTACAACTAAACAAAATTTTAGTGTACACAATCA GATAACTACACAATCAATTATACCCAGGATCCATGGGTCGACGAATCCCCGGGTTAATTAA TCCATGGAAGAGAAGATGGAAAAAGAATTTCATAGCCGTCTCAGCAGCCAACCGCTTTAAG AAAATCTCATCCTCCGGGGCACTTGATTATGATATTCCAACTACTGCTAGCGAGAATTTGT ATTTTCAGGGAGAATTCGGCCTTGCGCAACACGATGAAGCCGTGGACAACAAATTCAACAA AGAACAACAAAACGCGTTCTATGAGATCTTACATTTACCTAACTTAAACGAAGAACAACGA AACGCCTTCATCCAAAGTTTAAAAGATGACCCAAGCCAAAGCGCTAACCTTTTAGCAGAAG CTAAAAAGCTAAATGATGCTCAGGCGCCGAAAGTAGACAACAAATTCAACAAAGAACAACA AAACGCGTTCTATGAGATCTTACATTTACCTAACTTAAACGAAGAACAACGAAACGCCTTC ATCCAAAGTTTAAAAGATGACCCAAGCCAAAGCGCTAACCTTTTAGCAGAAGCTAAAAAGC TAAATGATGCTCAGGCGCCGAAAGTAGACGCGAATCATCAGTGAGGATCCTAAAGCTTTTC TAGTATGGTTTGAAACCTTACAATTTTTCTTCTTTGTTCCTTTTTCCTTGTTTCAGTGTAT ATTAGGTTGGGAAAGAGGGATTTTTCCATACCATATGACTGACTACAATATATACATGTAT AATAACTTCATAATCTAAACCTATCAGTTCAGTATCAAGTCAGCTATTCCGCCCTATGCAT AAACCTACAAACTATCATTCACACACTTTTCCCATTTTTTTTCAATACTACTTTACATCCG AACATTTTAGCAACCCACACCATATACCTTTGGTGCACTATTGATTTTCTTCCTGATGTCA GCTTTTTGTGCTTTGACAAAAAAATCGCGTCTACGTCCGTCCGTTCTCCCTGAATAAATTA GGCGCGTTTGAGCCCAGCAGGACGGAGCTCTAGTGACAAGCCCTGGTGTTTGGTGAGGTTT TGCACATTGCTGTTCTTTCTACTGTATTGAGATCTCCAGTTTACGGCTCCCTGGGAGCCAC CCGTAACGCGGTTGGTGTGCCCATTTCAATAAGCGAACATTAGTGAAGATACAATgcggcc gc ADH1: COX17 promotor is cloned between the XhoI and the BamHI site. The TAP-ORF (Red) and ADH1 3’UTR are cloned as a PCR product from BamHI to SacI. The AscI site is shown in green underlined text immediately downstream of the Stop-codon (TGA). ctcgaggtcgacGTCTTCACTGTCACTGATTGTAGGGACAAAGTCCAAATTAGAGTATTTT TTAGTTCCTACCACCATAGTATATATATAAGACGCTGTTGTAGGCACACAGCTCCAATTCG AAACCCAAGTTACCAACGTAAATACTCATACTATCTAAGCATATTCTAACTTACAATTTTC AACCTCATCTCATCGCATTTTTTGAAAATTTTTTTTCAAATCATTACCCGGATAATGTTAT AACCCTACCTATCTCCTTCAAAAAAAGATAAAATGACGATATATAGCGGCGAATCAGAATC TAGTAACCACCTAACTACTTGTCTCTAGTACAACTAAACAAAATTTTAGTGTACACAATCA GATAACTACACAATCAATTATACCCAGGATCCATGGGTCGACGAATCCCCGGGTTAATTAA TCCATGGAAGAGAAGATGGAAAAAGAATTTCATAGCCGTCTCAGCAGCCAACCGCTTTAAG AAAATCTCATCCTCCGGGGCACTTGATTATGATATTCCAACTACTGCTAGCGAGAATTTGT ATTTTCAGGGAGAATTCGGCCTTGCGCAACACGATGAAGCCGTGGACAACAAATTCAACAA AGAACAACAAAACGCGTTCTATGAGATCTTACATTTACCTAACTTAAACGAAGAACAACGA AACGCCTTCATCCAAAGTTTAAAAGATGACCCAAGCCAAAGCGCTAACCTTTTAGCAGAAG CTAAAAAGCTAAATGATGCTCAGGCGCCGAAAGTAGACAACAAATTCAACAAAGAACAACA AAACGCGTTCTATGAGATCTTACATTTACCTAACTTAAACGAAGAACAACGAAACGCCTTC ATCCAAAGTTTAAAAGATGACCCAAGCCAAAGCGCTAACCTTTTAGCAGAAGCTAAAAAGC TAAATGATGCTCAGGCGCCGAAAGTAGACGCGAATCATCAGTGAGGCGCGCCACTTCTAAA TAAGCGAATTTCTTATGATTTATGATTTTTATTATTAAATAAGTTATAAAAAAAATAAGTG TATACAAATTTTAAAGTGACTCTTAGGTTTTAAAACGAAAATTCTTATTCTTGAGTAACTC TTTCCTGTAGGTCAGGTTGCTTTCTCAGGTATAGTATGAGGTCGCTCTTATTGACCACACC TCTACCGGCAGATCCGCTAGGGATAACAGGGTAATATgagctc HSP12: Same as COX17, except that 3’UTR of HSP12 is inserted from BamHI to SacI. ctcgaggtcgacGTCTTCACTGTCACTGATTGTAGGGACAAAGTCCAAATTAGAGTATTTT TTAGTTCCTACCACCATAGTATATATATAAGACGCTGTTGTAGGCACACAGCTCCAATTCG AAACCCAAGTTACCAACGTAAATACTCATACTATCTAAGCATATTCTAACTTACAATTTTC AACCTCATCTCATCGCATTTTTTGAAAATTTTTTTTCAAATCATTACCCGGATAATGTTAT AACCCTACCTATCTCCTTCAAAAAAAGATAAAATGACGATATATAGCGGCGAATCAGAATC TAGTAACCACCTAACTACTTGTCTCTAGTACAACTAAACAAAATTTTAGTGTACACAATCA GATAACTACACAATCAATTATACCCAGGATCCATGGGTCGACGAATCCCCGGGTTAATTAA TCCATGGAAGAGAAGATGGAAAAAGAATTTCATAGCCGTCTCAGCAGCCAACCGCTTTAAG AAAATCTCATCCTCCGGGGCACTTGATTATGATATTCCAACTACTGCTAGCGAGAATTTGT ATTTTCAGGGAGAATTCGGCCTTGCGCAACACGATGAAGCCGTGGACAACAAATTCAACAA AGAACAACAAAACGCGTTCTATGAGATCTTACATTTACCTAACTTAAACGAAGAACAACGA AACGCCTTCATCCAAAGTTTAAAAGATGACCCAAGCCAAAGCGCTAACCTTTTAGCAGAAG CTAAAAAGCTAAATGATGCTCAGGCGCCGAAAGTAGACAACAAATTCAACAAAGAACAACA AAACGCGTTCTATGAGATCTTACATTTACCTAACTTAAACGAAGAACAACGAAACGCCTTC ATCCAAAGTTTAAAAGATGACCCAAGCCAAAGCGCTAACCTTTTAGCAGAAGCTAAAAAGC TAAATGATGCTCAGGCGCCGAAAGTAGACGCGAATCATCAGTGAGGATCCTAAATCTTTCG GAACTCTTTGTAGTTACATGGTTTTTTCTTTATGATGTGTGATGTTCCTTAATATTATATT CAATGAATAGCAGAGTAATAAAAAAATTCTAAAAAAAAAACGCTTAAATCGAGGTGGAATA TATAAACTTAAGTACGCATATACTCTAGTTCAGTTTAAAAATTAGCTGTTTTTTTAAAAAA AAAAATTCTCTTTCTTTACAAAATGTTCTTAAATGTATGTATGTGTGTGTGTGTGTGTGTG CGTATTGTTCTATTTCACTCCAGCTTAAACATGGCGGTTGCTTCTTCGTCATCAATTTCCG CGCGTTCGATTTTATTTAAAAGTACTTTCTTTGAAGCGCCGAGATTTTTCATGAAATCCGT CTCAAATCCAAGCAAATTATCTTTGAATTTCTTTCTCTTTTTACCGCGAATCCCAAGCACT GACGCTACGGGGATGTCTTCAAATTCATTGAATATCTTGATTAACCCAATATCGCGTCTTT TCAACCTGTTTGGCACCTGGGATTTTTTCAAATCGGCGAGAAACTCCTCTTGTGTCTTACC ATTTTCTGACACATGCTTGTTCAGATCTAGCGGAGCTC