Molecular Biology Laboratory
advertisement

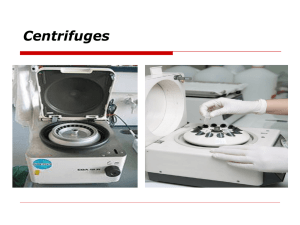
Lecture02 Molecular Biology Laboratory Prepared by Jawaher al-Zahrani Submitted To Dr. Maha Daghestani 1 Lecture02 Laboratory Safety Procedures for Molecular biology lab. A. GENERAL 1. Work carefully and cautiously in the laboratory, using common sense and good judgment at all times. 2. EATING AND DRINKING ARE PROHIBITED in the laboratory. 3. Long hair must be tied back during laboratory sessions. 4. Identify the location of all exits from the laboratory and from the building. 5. Keep sinks free of paper or any debris that could interfere with drainage. 6. Lab tables must be clear of all items that are not necessary for the lab exercise. 7. Wash hands and the lab tables with the appropriate cleaning agents before and after every laboratory session. 2 Lecture02 B. SHARP OBJECTS AND BROKEN GLASS 1. Pointed dissection probes, scalpels, razor blades, scissors, and microtome knives must be used with great care, and placed in a safe position when not in use. 2. Containers designated for the disposal of sharps (scalpel blades, razor blades, needles; dissection pins, etc.) and containers designated for broken glass are present in each laboratory. Never dispose of any sharp object in the regular trash containers. 3. Do not touch broken glass with bare hands. Put on gloves and use a broom and dustpan to clean up glass. Dispose of ALL broken glass in the specific container marked for glass. Do not place broken glass in the regular trash. E. INSTRUMENTS AND EQUIPMENT Care must be used when handling any equipment in the laboratory. Students are responsible for being familiar with and following correct safety practices for all instruments and equipment used in the laboratory. 3 Lecture02 G. BODY FLUIDS Special precautions are to be followed in all laboratories using any body fluids, such as blood, saliva, and urine, because of the potential to transmit disease-causing organisms. 1. Use gloves in all laboratory experiments that involve the use of body fluids. 2. All contaminated material, such as slides, coverslips, toothpicks, lancets, alcohol swabs, etc., must be placed in a biohazard bag for proper disposal and should never be reused. 4 Lecture02 Blood Collection Tubes EDTA TUBES: EDTA tube is widely used for clinical hematology as well as various kinds of blood cell test instrument. It makes use of EDTA as anticoagulant. Meanwhile, it offers a comprehensive protection for blood cell, especially for protecting the blood platelet, so that it can effectively stop the gathering of blood platelet and makes the form and volume of blood cell uninfluenced in a long time. EDTA tube can be used for DNA extraction and plasma isolation. HEPARIN TUBES: Heparin tube is used for blood collection and anti-coagulation, not only for routine clinical biochemistry tests and emergency biochemistry tests, but also for some test items in blood theology. It is coated with heparin. The anticoagulant heparin activates anti-thrombins, thus blocks the coagulation cascade and produces a whole blood/ plasma sample instead of clotted blood plus serum. In addition, this type of tubes can be used for cell culture NO ADDITIVE TUBES: No additive tube is used for blood collection and storage for biochemistry, immunology and serology tests in medical inspection. It can provide enough and nonpolluted serum specimen for clinical tests, while keeping the serum invariable in the long inspection period. It is applicable for all current mainstream biochemical analyzers 5 Lecture02 Isolation of Serum and Plasma Note: Blood Plasma contains many vital proteins including fibrinogen, globulins and human serum albumin. Serum refers to blood plasma in which clotting factors (such as fibrin) have been removed naturally by allowing the blood to clot prior to isolating the liquid component - Isolation of the Serum from Blood. 1. Collect the blood in Red top tubes and allow it to coagulate for 30-60 minetes. 2. Centrifuge at 3000 rpm for 15 minuets. 3. Transfer the serum to 1.5 ml tubes. - Isolation of the Plasma from Blood. 1. Collect the blood in EDTA (purple top) tubes. 2. Centrifuge at 3000 rpm for 15 minutes. 3. Transfer the Plasma to 1.5 ml tubes. 6 Lecture02 Principles of centrifugation Centrifuge performance can be classified as low speed, high-speed and ultra-speed. Usual applications include the separation of serum or plasma from red blood cells, the separation of precipitated solids from the liquid phase of a mixture, or the separation of liquids of varying densities. Particles suspended in a fluid move, under the influence of gravity, towards the bottom of a vessel at a rate that depends, in general, on their size and density. Centrifugation is a technique designed to utilise centrifugal forces, which are greater than the force of gravity, to speed up the sedimentation rate of particles. This is achieved by spinning the vessel containing the fluid and particles about an axis of rotation so that the particles experience a force acting away from the axis. The force is measured in multiples of the Earth’s gravitational force and is known as the relative centrifugal field (RCF) or, more commonly, the ‘g force’. Relative centrifugal field The RCF generated by a rotor depends on the speed of the rotor in revolutions per minute (rpm) and the radius of rotation (ie the distance from the axis of rotation). The equations that permit calculation of the RCF from a known rpm and radius of rotation and calculation of the rpm from a known RCF and radius are shown in Table 1. The RCF value can also be obtained using a nomogram. Using a straight-edged ruler, line up the known rotating radius (distance from the centre of rotor to the bottom of the centrifuge bucket) on the left with the known rpm on the far right and read the RCF value where the line crosses the graph in the centre. Most manufacturers include a nomogram in the instruction manual; however, most modern centrifuges now have the facility to swap the figure displayed on the control panel between rpm and RCF, making manual calculation unnecessary. 7 Lecture02 Table 1. Calculations used to convert rpm to RCF, and vice versa. Low-speed instruments Low-speed centrifuges have maximum rotor speeds of less than 10,000 rpm, which do not require the rotors to be run in vacuum, and there are instruments with a temperature control facility. Most instruments now include a sensor that will detect any imbalance when the rotor is running and cut off the drive mechanism if imbalance is present. Low-speed instruments are used to separate serum or plasma from red 8 Lecture02 blood cells, and to harvest and purify chemical precipitates, intact cells, nuclei, large mitrochondria and large plasma membrane fragments. High-speed instruments In general, high-speed centrifuges are capable of rotor speeds up to 21,000 rpm, although the new generation of super-speed instruments are capable of rotor speeds of 30,000 rpm, in which RCFs of 120,000 xg are possible. These instruments require refrigeration systems to overcome the heat generated by the friction of the spinning rotor, and the higher-speed machines must incorporate vacuum systems. High-speed centrifuges are used in the separation of a number of cell constituents and in the isolation and purification of viruses. Ultracentrifuges Ultracentrifuges are capable of speeds in excess of 30,000 rpm and RCFs of over 600,000 xg. They can be used in the isolation and purification of membrane components such as the endoplasmic reticulum and Golgi ,membrane, endosomes, ribosomes, DNA and RNA. Once again, refrigeration and vacuum systems are necessary. Instrument components Rotor The design of most centrifuges allows the drive system to accept rotors of different sizes and capacities, although most instrument rotors are now capable of accepting a large range of tube sizes through the use of adaptors. The load on the rotor should always be balanced before operating the centrifuge, particularly when using highspeed instruments in which the buckets and caps are often numbered so that they can be matched on opposite sides of the rotor. The load must be balanced both by equal mass and by centres of gravity across the centre of rotation. Safety lid Modern centrifuges must have a door-locking mechanism to prevent the lid from being opened while the instrument is running. If there is a power failure or the safety latch fails for some reason it may be necessary to trip the door-locking mechanism manually to retrieve the samples. Manufacturers’ instructions should be checked for the exact procedure required. 9 Lecture02 Refrigerator A centrifuge generates heat as it rotates and if samples are temperature labile then a refrigerated centrifuge should be used. Some centrifuges enable the rotor and chamber to be precooled before a run. 10