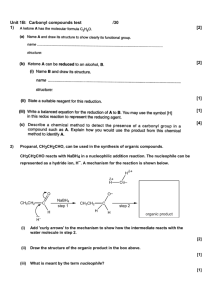
Practice Problems Mass Ratios Chapter 9_5
advertisement
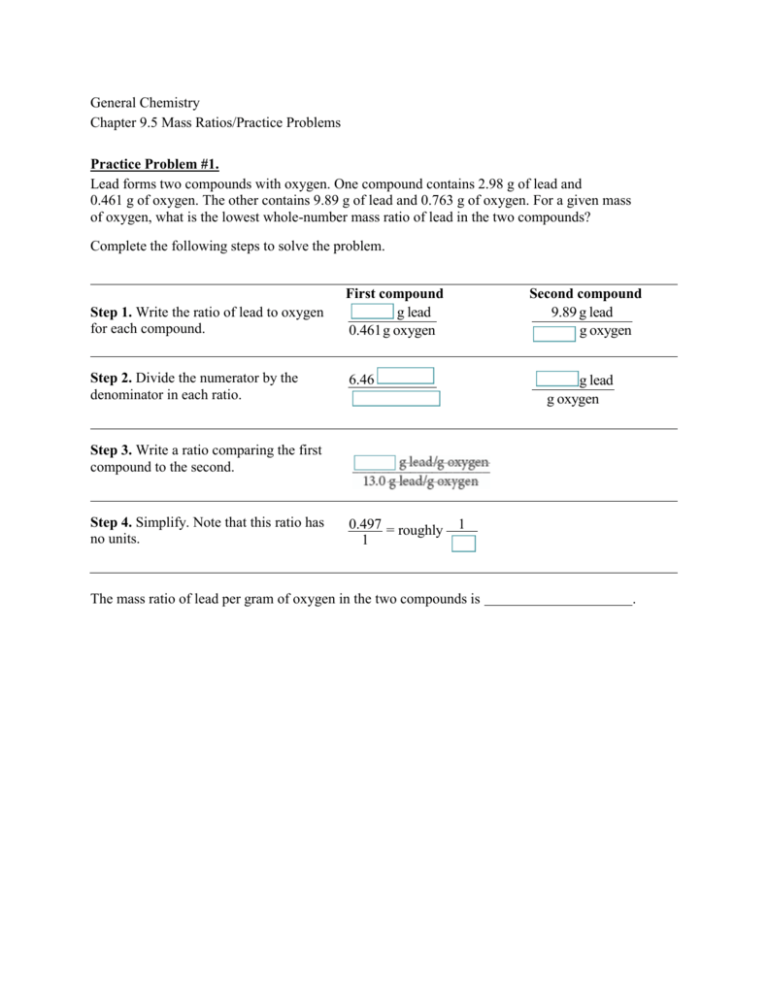
General Chemistry Chapter 9.5 Mass Ratios/Practice Problems Practice Problem #1. Lead forms two compounds with oxygen. One compound contains 2.98 g of lead and 0.461 g of oxygen. The other contains 9.89 g of lead and 0.763 g of oxygen. For a given mass of oxygen, what is the lowest whole-number mass ratio of lead in the two compounds? Complete the following steps to solve the problem. Step 1. Write the ratio of lead to oxygen for each compound. Step 2. Divide the numerator by the denominator in each ratio. First compound g lead 0.461 g oxygen Second compound 9.89 g lead g oxygen 6.46 g lead g oxygen Step 3. Write a ratio comparing the first compound to the second. Step 4. Simplify. Note that this ratio has no units. 0.497 = roughly 1 1 The mass ratio of lead per gram of oxygen in the two compounds is . Practice Problem #2 Magnesium reacts with oxygen to form two compounds. Compound A contains 7.88 g of magnesium for every 15.68 g of oxygen. Compound B contains 2.12 g of magnesium for every 6.91 g of oxygen. What is the mass ratio of magnesium rounded to the nearest whole number? Step 1. Write the ratio of magnesium to oxygen for each compound. First compound g Mg Step 2. Divide the numerator by the denominator in each ratio. (1) Step 3. Write a ratio comparing the first compound to the second. Second compound g Mg gO gO g Mg g Mg gO (1) g O g Mg/g O First Compound g Mg/g O Second Compound Step 4. Simplify. Note that this ratio has no units. The mass ratio of Mg per gram of oxygen in the two compounds is .