Kinetics
advertisement

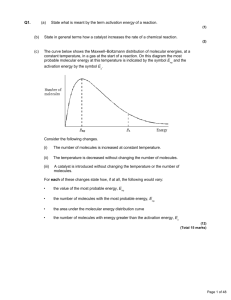
KINETICS Kinetics is concerned with the rate and mechanism of a chemical change. The rate of a chemical reaction is expressed as the change in the concentration of a reactant or product in unit time and usually has the unit mol.dm-3.s-1 Although a chemical equation shows a chemical change occurring in one step, most reactions are more complex and proceed in a number of steps, which occur at different rates. The slowest of these steps, irrespective of where it occurs, governs the overall rate of the reaction and is known as the rate-determining step. Collision Theory Before two substances can react with each other, their particles, which may be atoms, ions or molecules, must collide with each other. However, only a small proportion of the collisions which occur are successful i.e. they lead to a reaction. This is because, for a reaction to occur, particles must collide with sufficient energy and, in the case of more complex molecules, with the correct orientation. The activation energy is the minimum energy which colliding molecules must have to undergo a reaction. Maxwell-Boltzmann Distribution Curve In a sample of a gas or a liquid the molecules are in constant motion. They undergo elastic collisions with each other and with the walls of the container. An elastic collision is one in which kinetic energy is not dissipated in any other form of energy. Varying amounts of kinetic energy are transferred from one molecule to another during these collisions. The result is that, at a given temperature, the molecules in a sample have a range of energies. This distribution of energies is shown in a Maxwell-Boltzmann distribution curve: most common energy mean energy Number of molecules The area under the curve represents the total number of molecules in the sample Energy TOPIC 12.6: KINETICS 1 Points to notice: The curve starts at the origin, because there are no molecules with zero energy. Only a few particles have very high energies. At high energies, the curve is asymptotic, because there is no maximum energy for molecules. Factors Affecting Reaction Rate The rate of a chemical reaction is affected by: The concentration of reagents in solution The pressure of gaseous reagents The surface area (particle size) of solid reagents The temperature of the reaction mixture The presence of a catalyst 1. Concentration of a Solution Increasing the concentration of a reagent increases the number of particles in a given volume, and therefore increases the probability of a successful collision. The graph below shows the Maxwell-Boltzmann distribution curves for two samples at the same temperature. The shape of the curves is the same, but the more concentrated solution contains more particles, and therefore the area under the curve is greater. The particles which can undergo a successful collision are those with energy greater than or equal to the activation energy (E a). This number is greater for the more concentrated solution, therefore the rate is faster. Higher conc. Lower conc. Number of molecules Ea TOPIC 12.6: KINETICS 2 Energy As a reaction proceeds, the reactants are used up and their concentration therefore falls. This means that as time goes on, the chances of a successful collision diminish. In other words, the rate of the reaction is at its greatest at the start of the reaction and gradually falls as the reaction progresses. Concentration of reactant Time The rate of the reaction at any time is given by the gradient of this graph. 2. Pressure of a Gas There is no fundamental difference between an increase in the concentration of a solution and an increase in the pressure of a gas. Increasing the pressure forces the gas molecules closer together. Since there are more molecules in a given volume, the probability of a successful collision increases. All the arguments in section 1. are equally valid here. 3. The Surface Area (Particle Size) of a Solid Increasing the surface area of a solid increases the rate of reaction. When a solid reagent reacts, the reaction usually takes place only on the exposed surface of the solid, where the second reagent can collide with it. Therefore, if the solid is ground up, increasing its surface area (decreasing its particle size), this effectively increases its concentration and allows more successful collisions to occur with the second reagent. TOPIC 12.6: KINETICS 3 4. Temperature An increase in temperature always increases the rate of a chemical reaction. A useful rule of thumb is that increasing the temperature by 10oC roughly doubles the rate. When the temperature is increased, the average kinetic energy of the particles increases. This has two effects: The particles move faster and so make more collisions in a given time: this is a minor effect The proportion of particles with energy greater than or equal to the activation energy increases, so a larger proportion of collisions is successful. This is the major effect and is responsible for the rapid increase in rate as temperature increases. As temperature increases, the shape of the Maxwell-Boltzmann distribution curve changes. Since the sample is the same, the area under the curve is constant, but the curve broadens because there is a larger proportion of molecules with higher energies. The most common energy and the mean energy both increase, but the height of the maximum decreases. T1 T2 > T1 Number of molecules T2 Ea Energy The shaded areas represent the numbers of molecules with energy greater than or equal to the activation energy at T1 and T2. At T2 many more molecules have enough energy to react and therefore the rate is greater. TOPIC 12.6: KINETICS 4 5. Addition of a Catalyst A catalyst is a substance which alters the rate of a chemical reaction without itself being consumed in the reaction. Most catalysts are positive catalysts, which means that they increase the rate of a reaction. However, negative catalysts are used to slow down the rate of unwanted reactions. An example of a negative catalyst is ethanol, which is added to trichloromethane to slow down its degradation in air. A positive catalyst works by providing an alternative reaction path which has a lower activation energy. Ea bonds breaking Chemical Energy bonds forming The catalyst does not affect H for the reaction. For this reason, it does not affect the position of an equilibrium. Ea cat reactants H products Reaction path If the activation energy is reduced, more particles have energy greater than or equal to the activation energy, so the rate increases. The shaded areas below correspond to the numbers of particles which have enough energy to react in the catalysed and uncatalysed reactions. Number of molecules Ea cat TOPIC 12.6: KINETICS 5 Ea Energy