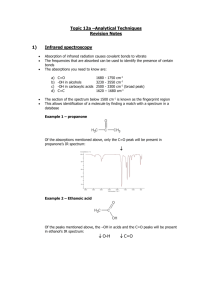
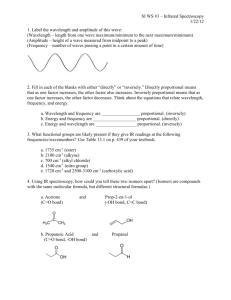
Document
advertisement

Spectroscopy: IR Renee Y. Becker Valencia Community College CHM 2011 • Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) • Different types are classified by frequency or wavelength ranges • γ-rays (gamma rays)- greatest energy and highest frequency. Emitted from some radioactive nuclei. Can cause biological damage. • X-rays- lower in energy than γ -rays. Can cause biological damage in high doses. • Ultraviolet (UV) light- can cause sunburn and even skin cancer. • Visible light- speaks for itself ! • Infrared radiation (IR) - heat! • Microwaves- we cook with them and also used in radar. • Radio waves- lowest frequency. Radio and TV transmissions and NMR spectroscopy. • The mode of propagation of electromagnetic radiation is the wave. • The wave is characterized by its wavelength (), frequency (), and amplitude. • Frequency () units: s-1 or hertz (Hz) • The intensity of a wave is proportional to the square of its amplitude. • Electromagnetic radiation travels at constant velocity in a vacuum: 3.00 x 1010 cm/s (speed of light). • Wavelength x Frequency = Speed • (cm) x (s-1) = c (cm/s) c c • Thanks to Max Planck and Al Einstein: hc E h E = Energy of 1 photon (1 quantum) h = Planck’s constant (6.62 x 10-34 J·s = 1.58 x 10-34cal·s) = Frequency (s-1) = Wavelength (cm) c = Speed of light (3.00 x 1010 cm/s) • The energy of a photon varies directly with the frequency and inversely with the wavelength • High frequencies and short wavelengths = higher energies • Low frequencies and long wavelengths = lower energies Infrared Spectroscopy of Organic Molecules • IR region lower energy than visible light (below red – produces heating as with a heat lamp) • 2.5 x 10-4 cm to 2.5 x 10-3 cm region used by organic chemists for structural analysis • IR energy in a spectrum is usually measured as wavenumber (cm-1), the inverse of wavelength and proportional to frequency • Specific IR absorbed by organic molecule related to its structure • The IR spectrum covers the range from 4000 cm-1 to 400 cm-1 • This represents energy ranges from 48.0 - 4.80 kJ/mol (11.5 - 1.15 kcal/mol). • IR energy absorption corresponds to specific modes, corresponding to combinations of atomic movements, such as bending and stretching of bonds between groups of atoms called “normal modes” • Energy is characteristic of the atoms in the group and their bonding • Corresponds to vibrations and rotations Interpreting Infrared Spectra • Most functional groups absorb at about the same energy and intensity independent of the molecule they are in • Characteristic higher energy IR absorptions in Table 12.1 can be used to confirm the existence of the presence of a functional group in a molecule • IR spectrum has lower energy region characteristic of molecule as a whole (“fingerprint” region; 1300 to 625 cm-1) • See samples in Figure 12-13 Regions of the Infrared Spectrum • 4000-2500 cm-1 N-H, CH, O-H (stretching) – 3300-3600 N-H, O-H – 3000 C-H • 2000-1500 cm-1 double bonds (stretching) – C=O 1680-1750 – C=C 1640-1680 cm-1 • 2500-2000 CC and C N (stretching) • Below 1500 cm-1 “fingerprint” region cm-1 Differences in Infrared Absorptions • Molecules vibrate and rotate in normal modes, which are combinations of motions (relates to force constants) • Bond stretching dominates higher energy modes • Light objects connected to heavy objects vibrate fastest: C-H, N-H, O-H • For two heavy atoms, stronger bond requires more energy: C C, C N > C=C, C=O, C=N > C-C, C-O, C-N, C-halogen Infrared Spectra of Hydrocarbons • C-H, C-C, C=C, C C have characteristic peaks – absence helps rule out C=C or C C IR: Alcohols and Amines • O–H 3400 to 3650 cm1 – Usually broad and intense • N–H 3300 to 3500 cm1 – Sharper and less intense than an O–H 1-butanol butylamine IR: Aromatic Compounds • Weak C–H stretch at 3030 cm1 • Weak absorptions 1660 - 2000 cm1 range • Medium-intensity absorptions 1450 to 1600 cm1 • See spectrum of phenylacetylene, Figure 12.15 IR: Carbonyl Compounds • Strong, sharp C=O peak 1670 to 1780 cm1 • Exact absorption characteristic of type of carbonyl compound – 1730 cm1 in saturated aldehydes – 1705 cm1 in aldehydes next to double bond or aromatic ring C=O in Ketones • 1715 cm1 in six-membered ring and acyclic ketones • 1750 cm1 in 5-membered ring ketones • 1690 cm1 in ketones next to a double bond or an aromatic ring 3-hexanone C=O in Esters • 1735 cm1 in saturated esters • 1715 cm1 in esters next to aromatic ring or a double bond Carboxylic Acids 1700 - 1725 cm O R -1 (strong) OH -1 2500 - 3300 cm (very broad and intense) Pentanoic acid Identify the functional groups in compounds that are responsible for the following absorptions: • A compound with a strong absorption at 1710 cm-1 • A compound with a strong absorption at 1540 cm-1 • A compound with a strong absorption at 1720 cm-1 and at 2500-3100 cm-1 How might you use IR spectroscopy to distinguish between the following pairs of isomers? • CH3CH2OH and CH3OCH3 • Cyclohexane and 1- hexene