IR/MS Key
advertisement

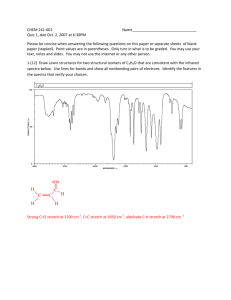
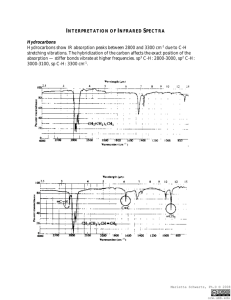
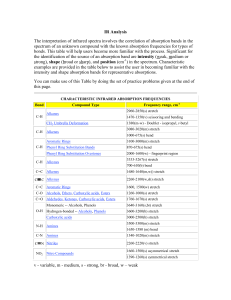
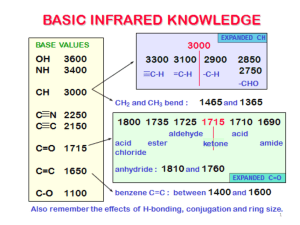
IR/MS Key Practice Problem 1 O-H stretch C=C triple bond stretch 3 sp C-H stretch 3 C H4O UN = (6+2-4)/2 = 2 2 C=O stretch m/z = 58 m/z = 43 m/z = 113 m/z = 128 (M) 3 UN = 0 Odd mass – contains N No N-H stretches Must be tertiary amine. m/z = 58 m/z = 73 (M) 4 From MS: contains Br From IR: carbonyl Two possibilities: m/z = 43 m/z = 150 (M) m/z = 107 • Propose structures for compounds that meet the following descriptions: a. An optically active compound C5H10O with an IR absorption at 1730 cm-1 b. A non-optically active compound C5H9N with an IR absorption at 2215 cm-1 a. UN = (12-10)/2 = 1 IR – must be carbonyl Only one optically active structure can be drawn: b. UN = (12-9+1)/2 = 2 IR – must be triple bond IR – no mention of N-H stretch 5 You are carrying out the dehydration of 1methylcyclohexanol to yield 1-methylcyclohexene. How could you use IR to determine when the reaction is complete? Look for: • Loss of O-H stretch ~3300 (broad) • Gain of C=C stretch ~1650 • Gain of sp2C-H stretch >3000 6 Propose a structure consistent with the following IR spectrum and molecular formula UN = (16-6)/2 = 5 sp2C-H C=O stretch aldehyde C-H C7H6O aromatic ring 7 • Nitriles undergo a hydrolysis reaction when heated with aqueous acid. What is the structure of the product of hydrolysis of propanenitrile (CH3CH2CN) if it has IR absorptions at 2500 to 3100 cm-1 (br) and 1710 cm-1 (s) and has a parent peak in MS at 74? 8 Propose a structure that is consistent with the following data: Mass spec shows a molecular ion at m/z = 69 m/z = 69 contains N Formula = C4H7N UN = 2 N From IR: triple bond (2246) No N-H 9 Propose a structure that is consistent with the following data: 10 Mass spec shows a molecular ion at m/z = 98 O From IR: C=O (1716) No aldehyde CHO MW 98 C6H10O UN = 2 Back to IR – no alkene (1650) must be ring