Supplementary Material Synthesis and Evaluation of Thermoplastic
advertisement
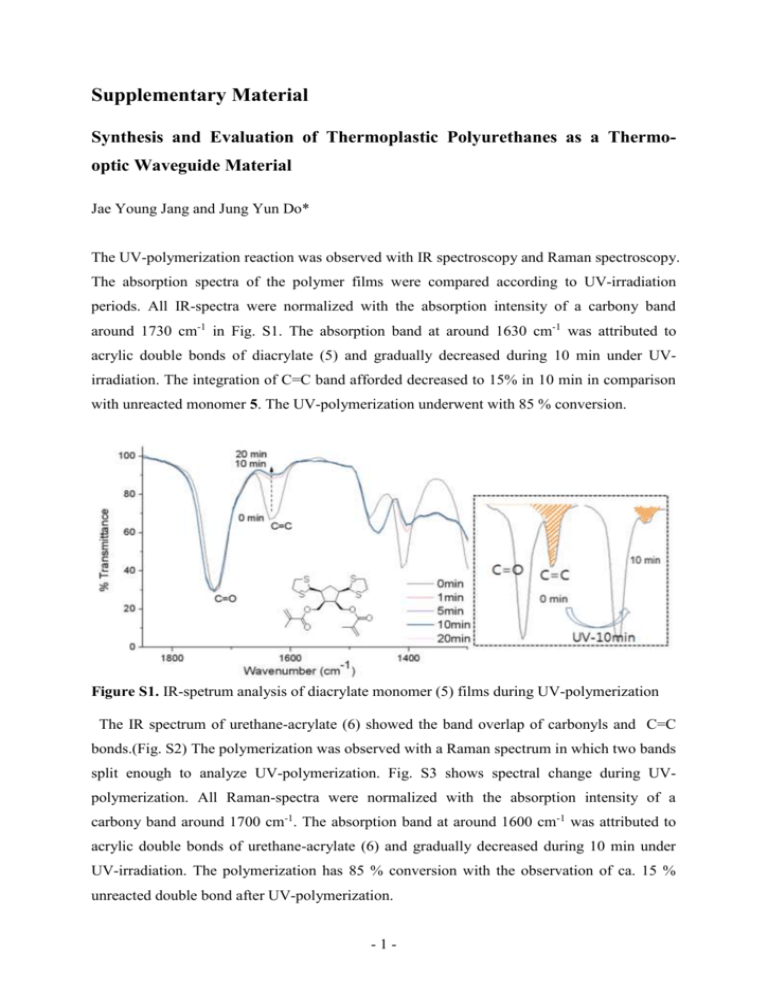
Supplementary Material Synthesis and Evaluation of Thermoplastic Polyurethanes as a Thermooptic Waveguide Material Jae Young Jang and Jung Yun Do* The UV-polymerization reaction was observed with IR spectroscopy and Raman spectroscopy. The absorption spectra of the polymer films were compared according to UV-irradiation periods. All IR-spectra were normalized with the absorption intensity of a carbony band around 1730 cm-1 in Fig. S1. The absorption band at around 1630 cm-1 was attributed to acrylic double bonds of diacrylate (5) and gradually decreased during 10 min under UVirradiation. The integration of C=C band afforded decreased to 15% in 10 min in comparison with unreacted monomer 5. The UV-polymerization underwent with 85 % conversion. Figure S1. IR-spetrum analysis of diacrylate monomer (5) films during UV-polymerization The IR spectrum of urethane-acrylate (6) showed the band overlap of carbonyls and C=C bonds.(Fig. S2) The polymerization was observed with a Raman spectrum in which two bands split enough to analyze UV-polymerization. Fig. S3 shows spectral change during UVpolymerization. All Raman-spectra were normalized with the absorption intensity of a carbony band around 1700 cm-1. The absorption band at around 1600 cm-1 was attributed to acrylic double bonds of urethane-acrylate (6) and gradually decreased during 10 min under UV-irradiation. The polymerization has 85 % conversion with the observation of ca. 15 % unreacted double bond after UV-polymerization. -1- Figure S2. IR-spetrum of urethane-acrylate (6) Figure S3. Raman-spetrum analysis of urethane-acrylate (6) films during UV-polymerization -2-