7 Classification of Matter-S
advertisement

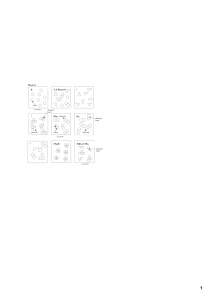
Classification of Matter How do atoms combine to make different types of matter? Why ? Look at the things in this room. They are all matter. That matter may be pure or it may be a mixture. Can you tell by looking at it? What if you looked at it under a microscope? Then could you tell? Something that looks pure may not really be pure. It depends on what type of particles an object or substance is made of. In this activity we will explore how the smallest chemical units of matter determine whether something is classified as an element, a compound, or a mixture. Model 1 — Atoms, Particles, and Molecules T & RSq & R R ? atom 8 partic les ? molecule ? chemi cal bond RSq molec ule ato ms 5 particles TSq2R chemi cal bond Sq2 molec ule SqR3 & TSq chemical bond Classification of Matter 1 5 particles 2 POGIL™ Activities for High School Chemistry 1. Locate the circled molecule of RSq in Model 1. a. Find a second RSq molecule and circle it. b. How many atoms are in a molecule of RSq? 2. Find and circle a molecule of TSq2R in Model 1. a. How many different types of atoms are found in a molecule of TSq2R? b. How many Sq atoms are in a molecule of TSq2R? 3. Locate the drawing labeled SqR3 & TSq in Model 1. a. How many different types of atoms are found in the sample of SqR3 & TSq? b. How many different types of molecules are found in the sample of SqR3 & TSq? 4. When two atoms are touching in the drawings of Model 1, what is holding the atoms together? 5. As a group, discuss the following questions and record your answers: a. Can a particle be a single atom? b. Can a particle be a molecule? c. How many particles are in the drawing representing T & RSq & R in Model 1? d. What is your group’s definition of the word “particle” as it is used in chemistry? 6. Compare the codes listed at the top of each drawing in Model 1 with the shapes in that box. a. What do the letters R, Sq, and T in the codes represent? b. What do the small numbers (subscripts) in the codes represent? c. When atoms are touching, how is that communicated in the code? Classification of Matter 3 d. What is the common characteristic of the samples in which an ampersand (&) is used? e. In Model 1 there are three drawings that are labeled with a question mark. Write codes to properly label these drawings. 4 POGIL™ Activities for High School Chemistry 7. Appoint one group member to cut apart Model 1 to separate the nine drawings. As a team, sort the drawings into two groups— one group where all the particles in the drawing are identical, and a second group in which the drawings contain more than one type of particle. Read This! Matter is classified as a pure substance when all of the particles are identical. Matter is classified as a mixture if there are different types of particles present. 8. Identify which drawings from Question 7 are pure substances and which are mixtures. List the codes for the drawings in the appropriate places below. Pure Substances Mixtures 9. How are the codes (chemical formulas) for pure substances different from those for mixtures? 10. As a team, take the set of pure substances drawings from Question 8 and sort them into two new groups, those containing only one type of atom and those with two or more types of atoms. Read This! Elements are defined as pure substances made from only one type of atom. Compounds are defined as pure substances made from two or more types of atoms. 11. Identify which drawings from Question 10 are elements and which are compounds. List the codes for the drawings in the appropriate places below. Elements Compounds 12. How are the codes (chemical formulas) for elements different from those for compounds? Classification of Matter 5 13. Use what you have just learned about chemical formulas to identify each of the following as an element, a compound or a mixture. a. Br2 b. NaHCO3 c. C6H12O6 & H2O d. Cu & Zn 6 e. CO2 f. Al POGIL™ Activities for High School Chemistry 14. Explain the difference between: a. An atom and an element. b. A molecule and a compound. Classification of Matter 7 Extension Questions 15. It is often useful to separate matter. Physical methods of separation (filtering, distillation) do not require a chemical change. In other words, no chemical bonds are broken or formed during the separation. Chemical methods of separation (decomposition, electrolysis) require a chemical change. In other words, chemical bonds are broken and/or formed during the separation. a. Is straining cooked pasta from water a physical or chemical separation? b. Is using a fuel cell to separate water into hydrogen and oxygen a physical or chemical separation? c. Which type(s) of matter (mixtures/compounds/elements) could be separated by physical methods? d. Which type(s) of matter (mixtures/compounds/elements) would need to be separated by chemical methods? 16. Students in a chemistry course were asked the following question on a unit exam: “Draw a dia- gram representing an element using circles as atoms.” a. The following diagrams represent two typical answers given by students. Which drawing is the best representation of an element? Explain. Drawing A Drawing B b. Imagine that the atom in Drawing B had been removed by physical separation from one of the substances in Model 1. What substances could have been the source of the atom in Drawing B? 8 POGIL™ Activities for High School Chemistry