Notes - NHS-AP
advertisement
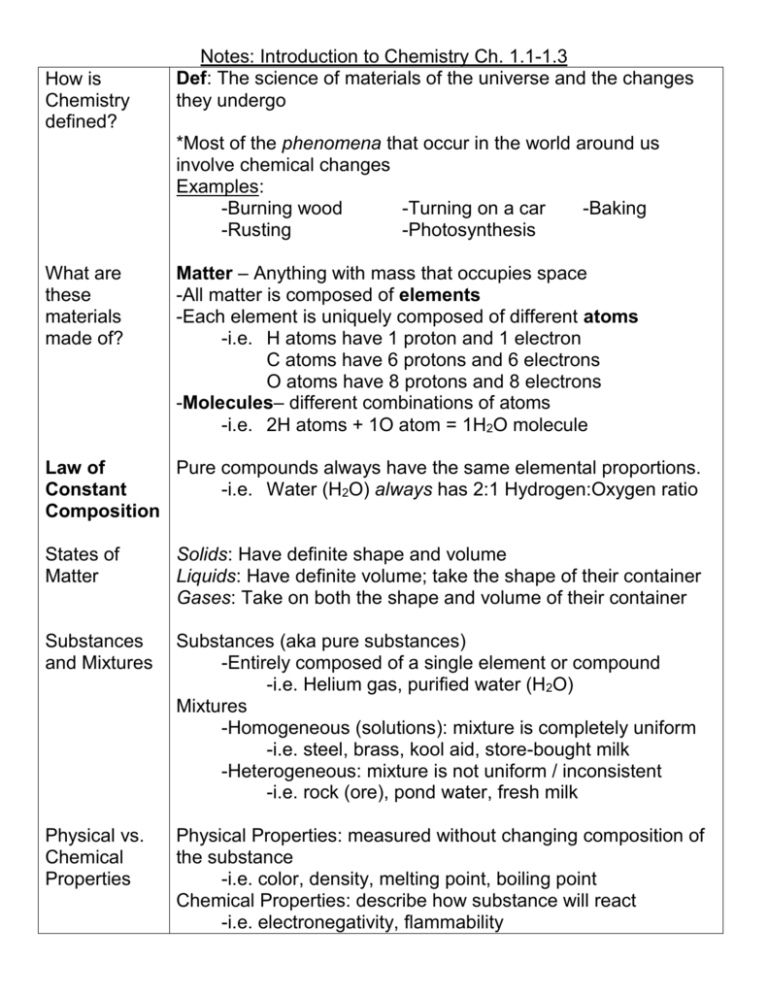
How is Chemistry defined? Notes: Introduction to Chemistry Ch. 1.1-1.3 Def: The science of materials of the universe and the changes they undergo *Most of the phenomena that occur in the world around us involve chemical changes Examples: -Burning wood -Turning on a car -Baking -Rusting -Photosynthesis What are these materials made of? Matter – Anything with mass that occupies space -All matter is composed of elements -Each element is uniquely composed of different atoms -i.e. H atoms have 1 proton and 1 electron C atoms have 6 protons and 6 electrons O atoms have 8 protons and 8 electrons -Molecules– different combinations of atoms -i.e. 2H atoms + 1O atom = 1H2O molecule Law of Pure compounds always have the same elemental proportions. Constant -i.e. Water (H2O) always has 2:1 Hydrogen:Oxygen ratio Composition States of Matter Solids: Have definite shape and volume Liquids: Have definite volume; take the shape of their container Gases: Take on both the shape and volume of their container Substances and Mixtures Substances (aka pure substances) -Entirely composed of a single element or compound -i.e. Helium gas, purified water (H2O) Mixtures -Homogeneous (solutions): mixture is completely uniform -i.e. steel, brass, kool aid, store-bought milk -Heterogeneous: mixture is not uniform / inconsistent -i.e. rock (ore), pond water, fresh milk Physical vs. Chemical Properties Physical Properties: measured without changing composition of the substance -i.e. color, density, melting point, boiling point Chemical Properties: describe how substance will react -i.e. electronegativity, flammability Physical Changes vs. Chemical Changes Practice: Physical Changes: Appearances change, but chemical composition stays the same. All state changes (solid, liquid, gas) are physical. -i.e. Ice, Water and Water Vapor are all H2O Chemical Changes: Substances transformed chemically into other substances -i.e. Hydrogen gas burns in oxygen to create water -2H2 + O2 2H20 Element, Molecule or Compound? -Au -CO2 -CH4 -N2 Physical or Chemical? -A tree is cut down and turned into lumber -The statue of liberty turning green -Copper is drawn into small wires for electronics -Plants converting sunlight into usable, stored energy